I100
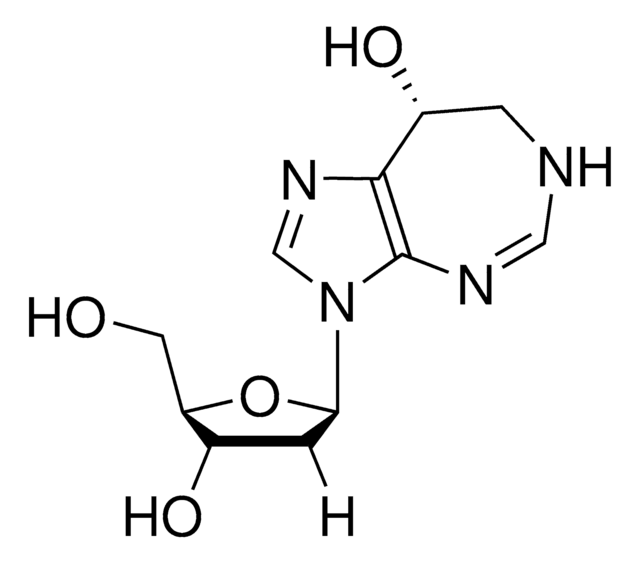
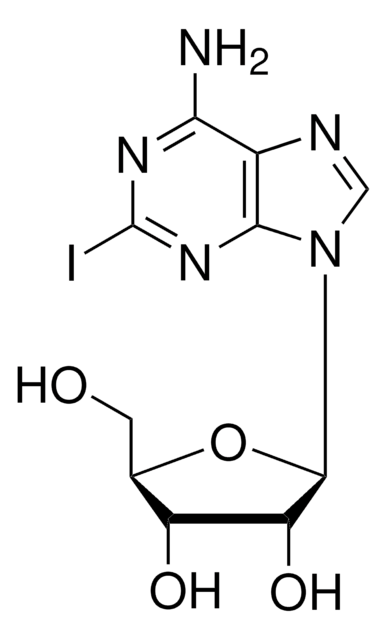
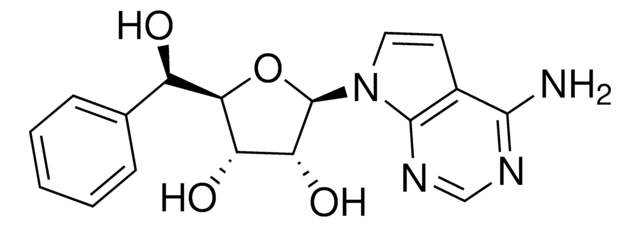
5-Iodotubercidin
≥85%, solid
Sinónimos:
4-Amino-5-iodo-7-(β-D-ribofuranosyl)pyrrolo[2,3-d]pyrimidine, 5-Iodotubericidin, 7-Iodo-7-deazaadenosine
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥85%
Formulario
solid
temp. de almacenamiento
2-8°C
cadena SMILES
Nc1ncnc2n(cc(I)c12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O
InChI
1S/C11H13IN4O4/c12-4-1-16(10-6(4)9(13)14-3-15-10)11-8(19)7(18)5(2-17)20-11/h1,3,5,7-8,11,17-19H,2H2,(H2,13,14,15)/t5-,7-,8-,11-/m1/s1
Clave InChI
WHSIXKUPQCKWBY-IOSLPCCCSA-N
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Precaución
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico