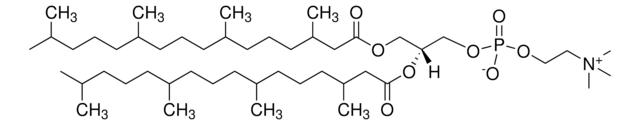
H9395
α-Hemolysin from Staphylococcus aureus
lyophilized powder, Protein ~60 % by Lowry, ≥10,000 units/mg protein
Sinónimos:
α-Toxin
About This Item
Productos recomendados
origen biológico
Staphylococcus aureus
Nivel de calidad
Formulario
lyophilized powder
actividad específica
≥10,000 units/mg protein
contiene
sodium citrate buffer as balance
composición
Protein, ~60% Lowry
solubilidad
H2O: soluble 0.49-0.51 mg/mL
Nº de acceso UniProt
temp. de almacenamiento
2-8°C
Información sobre el gen
Staphylococcus aureus ... SAOUHSC_01121(3920722)
Descripción general
Aplicación
- as a component of electrolyte solution for testing pore formation in lipid bilayer using electrophysiological measurements
- to test its osteogenesis suppressive effects in bone marrow stromal cells (BMSCs)
- in the preparation of α-hemolysin molecular imprinted polymer (MIP) for Biacore and surface plasmon resonance
Acciones bioquímicas o fisiológicas
Envase
Definición de unidad
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 2
Órganos de actuación
Lungs,Blood
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Contenido relacionado
An overview of cell lysis and protein extraction methods including detergent solubilization, freeze-thaw lysis, osmotic shock, sonication, enzymatic cell lysis, and mechanical disruption techniques such as Dounce, Polytron, and mortar and pestle homogenization.
An overview of cell lysis and protein extraction methods including detergent solubilization, freeze-thaw lysis, osmotic shock, sonication, enzymatic cell lysis, and mechanical disruption techniques such as Dounce, Polytron, and mortar and pestle homogenization.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico