G5660
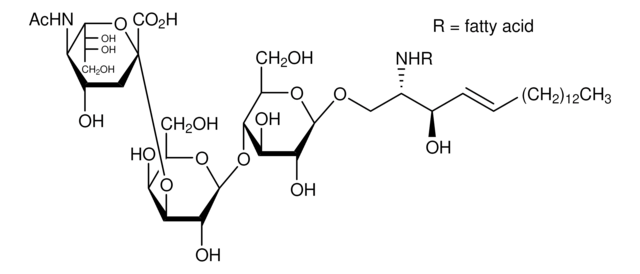
Lysoganglioside-GM1 from bovine brain
≥95%, lyophilized powder
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Productos recomendados
Categorías relacionadas
Acciones bioquímicas o fisiológicas
Suggested to be a modulator of cell growth and signal transduction. Accumulates in the nervous system of patients with GM1 gangliosidosis. Used for the preparation of GM1 derivatives containing different fatty acids, fluorescent tags, or other residues.
Otras notas
Gangliosides are major constituents of neuronal cell membranes and endoplasmic reticulum; contain a sialated polysaccharide chain linked to ceramide through a β-glycosidic linkage; for classification of gangliosides see Svennerholm, L., et al. (eds.), Structure and Function of Gangliosides, New York, Plenum, 1980.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
A Kharlamov et al.
Proceedings of the National Academy of Sciences of the United States of America, 91(14), 6303-6307 (1994-07-05)
A bilateral photochemically induced thrombotic lesion of rat sensorimotor cortex (approximately 3 mm in diameter and 25 mm3 in volume) is associated with a persistent cognition (learning and memory) deficit, which was evaluated with water maze tasks. The N-dichloroacetylsphingosine derivative
Y A Hannun et al.
Science (New York, N.Y.), 235(4789), 670-674 (1987-02-06)
Lysosphingolipids potently and reversibly inhibited protein kinase C activity and binding of phorbol dibutyrate in vitro and in human platelets. As with activation of protein kinase C by phosphatidylserine and sn-1,2-diacylglycerol, inhibition was subject to surface dilution. Accordingly, inhibition in
T Kobayashi et al.
Journal of neurochemistry, 59(4), 1452-1458 (1992-10-01)
By using a sensitive method, we assayed lysocompounds of gangliosides and asialogangliosides in tissues from four patients with GM2 gangliosidosis (one with Sandhoff disease and three with Tay-Sachs disease) and from three patients with GM1 gangliosidosis [one with infantile type
Tubaro, E., et al.
Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. Soc., 248, 175-175 (1993)
R T Huang et al.
FEBS letters, 281(1-2), 39-42 (1991-04-09)
Fluorescent dansyl derivatives of 3 natural sphingolipids (gangliosides, cerebroside and sphingomyelin) were shown to be readily taken up by culture cells (HeLa-, MDCK- and primary rat brain cells). A part of the incorporated fluorescent sphingolipids remained associated with the cells
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico