G0535
Glycopeptidase A from almonds
buffered aqueous glycerol solution, ≥0.05 unit/mL
Sinónimos:
N-Glycosidase A, N-linked-glycopeptide-(N-acetyl-β-D-glucosaminyl)-L-asparagine amidohydrolase, PNGase A
About This Item
Productos recomendados
conjugado
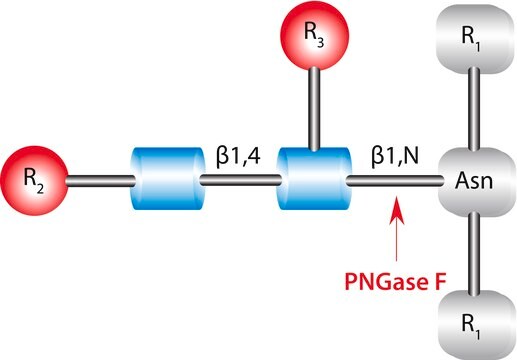
(N-linked)
Nivel de calidad
formulario
buffered aqueous glycerol solution
concentración
≥0.05 unit/mL
temp. de almacenamiento
−20°C
Categorías relacionadas
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Definición de unidad
Forma física
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
No data available
Punto de inflamabilidad (°C)
No data available
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
N-Linked Glycan Strategies; Sigma-Aldrich.com
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico