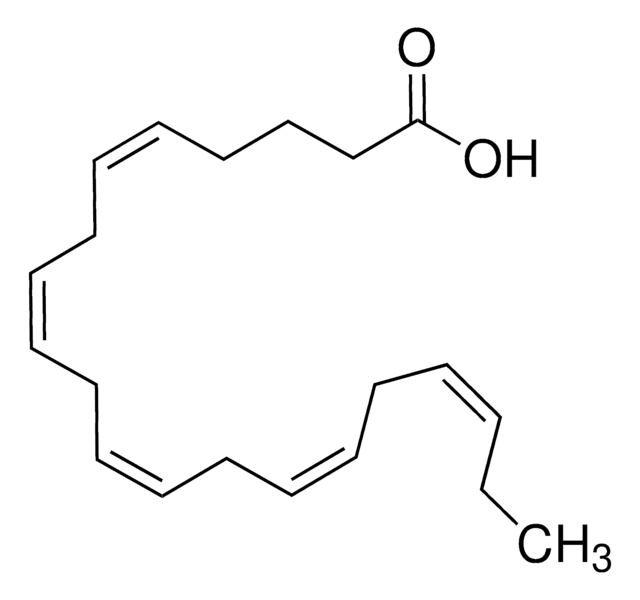
D8768
cis-4,7,10,13,16,19-Docosahexaenoic acid sodium salt
≥95%, waxy solid
Sinónimos:
Sodium (all-Z)-4,7,10,13,16,19-docosahexaenoate
About This Item
Productos recomendados
origen biológico
cod liver oil
Nivel de calidad
Análisis
≥95%
formulario
waxy solid
grupo funcional
carboxylic acid
tipo de lípido
omega FAs
Condiciones de envío
ambient
temp. de almacenamiento
−20°C
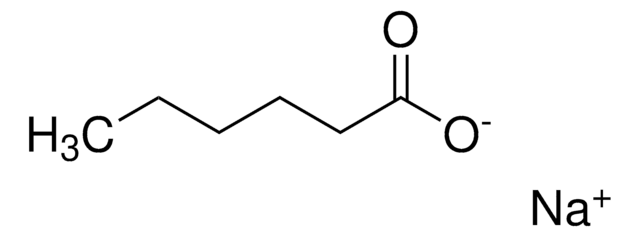
cadena SMILES
CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(O[Na])=O
InChI
1S/C22H32O2.Na/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24;/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24);/q;+1/p-1/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18-;
Clave InChI
SNNDEWVSGZRIFE-FPYKSTABSA-M
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The potential for the prevention and treatment of cardiovascular disease through increased dietary intake of omega-3 (w-3) fish oils is not a recent scientific discovery.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico