A3611
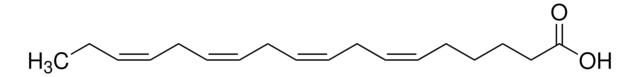
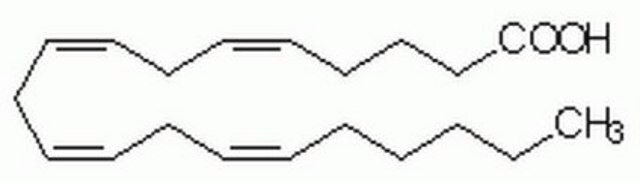
Arachidonic acid
from non-animal source, ≥98.5% (GC)
Sinónimos:
cis,cis,cis,cis-5,8,11,14-Eicosatetraenoic acid, Eicosa-5Z,8Z,11Z,14Z-tetraenoic acid, Immunocytophyte
About This Item
Productos recomendados
origen biológico
non-animal source
Nivel de calidad
Ensayo
≥98.5% (GC)
Formulario
liquid
índice de refracción
n20/D 1.4872 (lit.)
bp
169-171 °C/0.15 mmHg (lit.)
mp
−49 °C (lit.)
densidad
0.922 g/mL at 25 °C (lit.)
grupo funcional
carboxylic acid
tipo de lípido
omega FAs
Condiciones de envío
dry ice
temp. de almacenamiento
−20°C
cadena SMILES
OC(CCC/C=C\C/C=C\C/C=C\C/C=C\CCCCC)=O
InChI
1S/C20H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-19H2,1H3,(H,21,22)/b7-6-,10-9-,13-12-,16-15-
Clave InChI
YZXBAPSDXZZRGB-DOFZRALJSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
<li><strong>Molecular Mechanisms Associated with the Inhibitory Role of Long Chain n-3 PUFA in Colorectal Cancer:</strong> This study discusses the effects of long-chain polyunsaturated fatty acids, like arachidonic acid, on colorectal cancer mechanisms. The research focuses on the anti-inflammatory and cancer inhibitory roles through the modulation of lipid metabolism and signal transduction pathways (Jayathilake et al., 2024).</li>
<li><strong>Zhilining Formula alleviates DSS-induced colitis through suppressing inflammation and gut barrier dysfunction via the AHR/NF-Bp65 axis:</strong> This article presents arachidonic acid′s role in the suppression of inflammation and restoration of gut barrier function, crucial for understanding inflammatory diseases and developing therapeutic strategies (Zhou et al., 2024).</li>
<li><strong>5,6-diHETE lactone (EPA-L) mediates hypertensive microvascular dilation by activating the endothelial GPR-PLC-IP(3) signaling pathway:</strong> Explores the cardiovascular implications of arachidonic acid metabolites, specifically their role in microvascular responses, which could influence hypertension treatment strategies (Asulin et al., 2024).</li>
</ul>
Acciones bioquímicas o fisiológicas
Envase
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
235.4 °F - closed cup
Punto de inflamabilidad (°C)
113 °C - closed cup
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico