C0400
Carmustine
≥98% (TLC), oily liquid to amorphous solid, DNA alkylating agent
Sinónimos:
1,3-Bis(2-chloroethyl)-1-nitrosourea, BCNU
About This Item
Productos recomendados
Nombre del producto
Carmustine, ≥98%
Nivel de calidad
Ensayo
≥98%
Formulario
(Oily liquid to amorphous solid)
mp
30 °C (lit.)
solubilidad
ethanol: 19.60-20.40 mg/mL, clear, pale yellow to yellow
emisor
Bristol-Myers Squibb
temp. de almacenamiento
−20°C
cadena SMILES
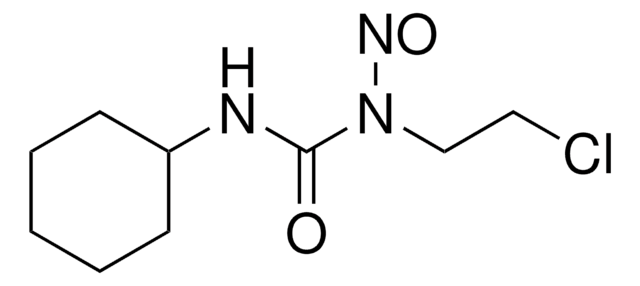
ClCCNC(=O)N(CCCl)N=O
InChI
1S/C5H9Cl2N3O2/c6-1-3-8-5(11)10(9-12)4-2-7/h1-4H2,(H,8,11)
Clave InChI
DLGOEMSEDOSKAD-UHFFFAOYSA-N
Información sobre el gen
human ... GSR(2936)
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Características y beneficios
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 2 Oral - Carc. 1B - Repr. 1B
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Contenido relacionado
Apoptosis, or programmed cell death (PCD), is a selective process for the removal of unnecessary, infected or transformed cells in various biological systems. As it plays a role in the homeostasis of multicellular organisms, apoptosis is tightly regulated through two principal pathways by a number of regulatory and effector molecules.
n proliferating cells, the cell cycle consists of four phases. Gap 1 (G1) is the interval between mitosis and DNA replication that is characterized by cell growth. Replication of DNA occurs during the synthesis (S) phase, which is followed by a second gap phase (G2) during which growth and preparation for cell division occurs. Together, these three stages comprise the interphase phase of the cell cycle. Interphase is followed by the mitotic (M) phase.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico