B3438
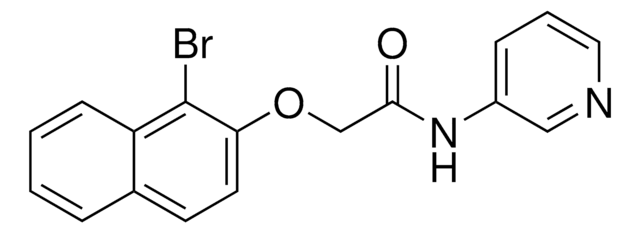
Pyrabactin
≥98% (HPLC)
Sinónimos:
4-Bromo-N-(2-pyridinylmethyl)-1-napthalenesulfonamide, 4-Bromo-N-(pyridin-2-ylmethyl)naphthalene-1-sulfonamide
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥98% (HPLC)
Formulario
powder
color
white to off-white
solubilidad
DMSO: >10 mg/mL
temp. de almacenamiento
room temp
cadena SMILES
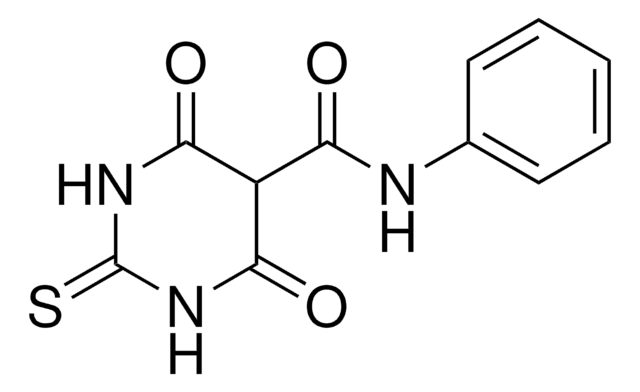
Brc1ccc(c2ccccc12)S(=O)(=O)NCc3ccccn3
InChI
1S/C16H13BrN2O2S/c17-15-8-9-16(14-7-2-1-6-13(14)15)22(20,21)19-11-12-5-3-4-10-18-12/h1-10,19H,11H2
Clave InChI
GJSDYQXOSHKOGX-UHFFFAOYSA-N
Aplicación
Acciones bioquímicas o fisiológicas
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Oral - Eye Irrit. 2
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole 98%](/deepweb/assets/sigmaaldrich/product/structures/181/460/3d58bc34-1b5c-4295-bbac-3b52085670e8/640/3d58bc34-1b5c-4295-bbac-3b52085670e8.png)