300764
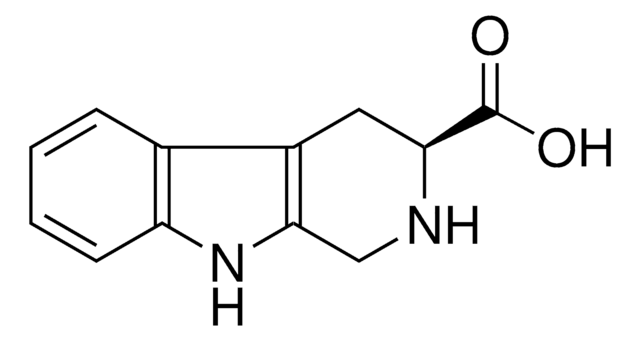
1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole
98%
Sinónimos:
Noreleagnine, THBC, Tetrahydronorharman, Tryptoline
About This Item
Productos recomendados
Nivel de calidad
Análisis
98%
formulario
liquid
mp
206-208 °C (lit.)
cadena SMILES
C1Cc2c(CN1)[nH]c3ccccc23
InChI
1S/C11H12N2/c1-2-4-10-8(3-1)9-5-6-12-7-11(9)13-10/h1-4,12-13H,5-7H2
Clave InChI
CFTOTSJVQRFXOF-UHFFFAOYSA-N
Información sobre el gen
rat ... Htr2a(29595) , Htr2c(25187)
Descripción general
Aplicación
- Reactant for synthesis of the indolyl-β-carboline alkaloid eudistomin U via IBX mediated room temperature oxidative aromatization
- Reactant for preparation of neuroprotective HDAC6 inhibitors
- Reactant for preparation of aminofuranopyrimidines as EGFR and Aurora A kinase inhibitors
- Reactant for preparation of inhibitors of CDK4
- Reactant for preparation of tetrahydrocarboline derivatives of as human 5-HT5A receptor ligands
- Reactant for preparation of 5-(diaminomethyl)-2,4-aminopyrimidines as dihydrofolate reductase inhibitors and antibacterial agents
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
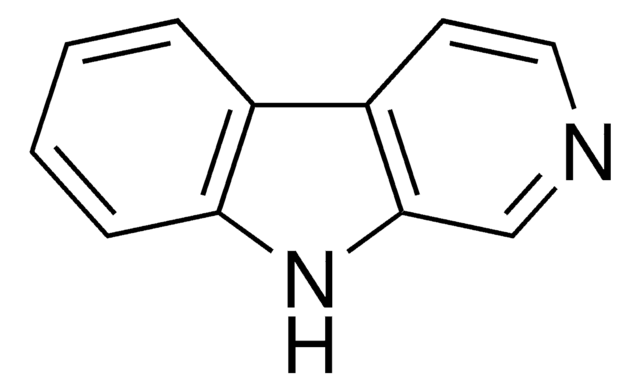
![2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/376/664/07577eb6-6e8c-4237-b8c5-03da4c8e7d88/640/07577eb6-6e8c-4237-b8c5-03da4c8e7d88.png)