78832
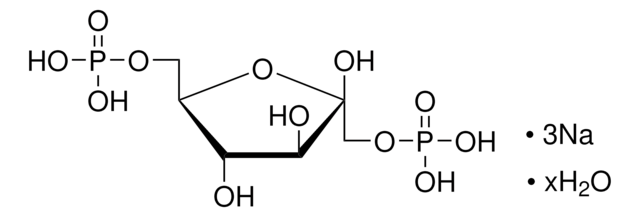
D-Sedoheptulose 7-phosphate lithium salt
≥90% (TLC)
Sinónimos:
D-altro-Heptulose 7-phosphate lithium salt
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
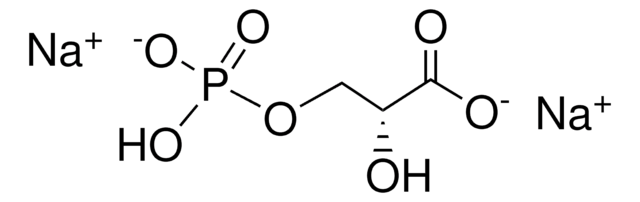
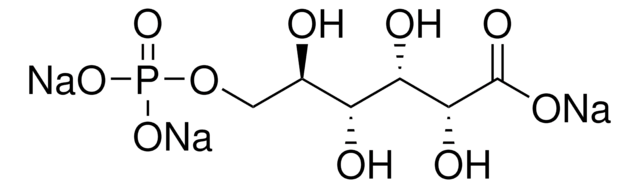
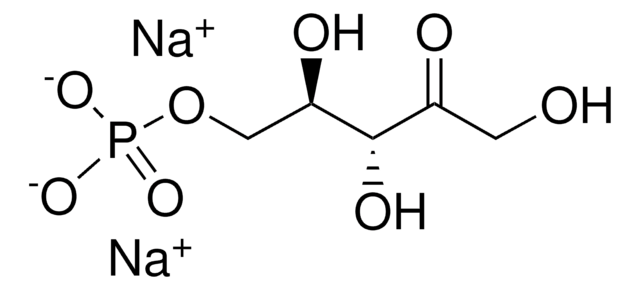
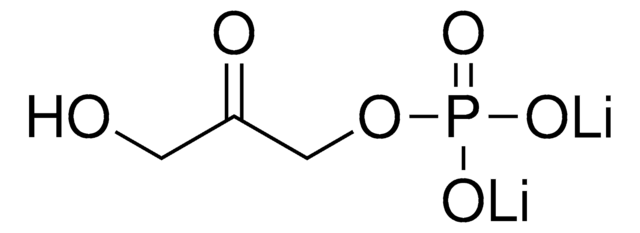
Fórmula empírica (notación de Hill):
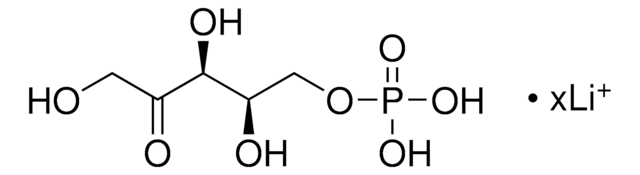
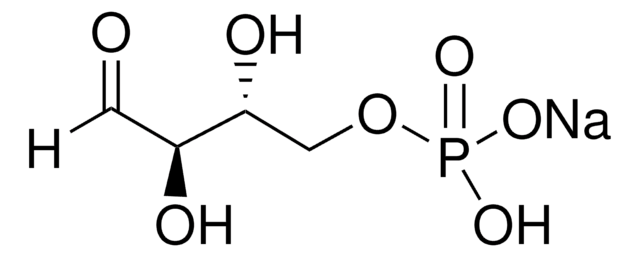
C7H15O10P · xLi+
Número de CAS:
Peso molecular:
290.16 (free acid basis)
Número MDL:
Código UNSPSC:
12352201
NACRES:
NA.25
Productos recomendados
Nivel de calidad
Ensayo
≥90% (TLC)
Formulario
powder
color
white to light yellow
temp. de almacenamiento
−20°C
cadena SMILES
[P](=O)(OC[C@@H](O)[C@@H](O)[C@@H](O)[C@H](O)C(=O)CO)(O)O
InChI
1S/C7H15O10P/c8-1-3(9)5(11)7(13)6(12)4(10)2-17-18(14,15)16/h4-8,10-13H,1-2H2,(H2,14,15,16)/t4-,5-,6-,7+/m1/s1
Clave InChI
JDTUMPKOJBQPKX-GBNDHIKLSA-N
Acciones bioquímicas o fisiológicas
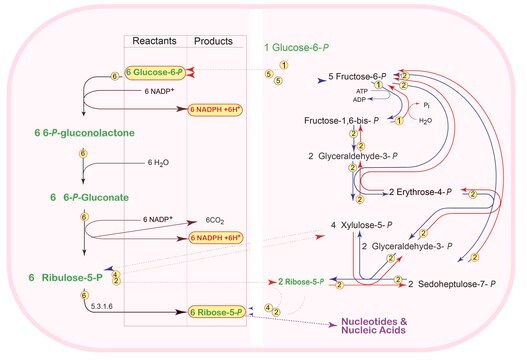
D-Sedoheptulose 7-phosphate is a metabolite in a number of pathways, e.g. an intermediate in the pentose phosphate pathway, and the carbon fixation in photosynthetic organisms.
D-Sedoheptulose is a pentose phosphate pathway (PPP) intermediate. D-Sedoheptulose-7-phosphate contributes to the generation of NADPH and the formation of ribose residues of nucleotide synthesis. Sedoheptulose-7-phosphate levels may be increased in individuals with defects in transaldolase (TALSDO1).
Envase
Bottomless glass bottle. Contents are inside inserted fused cone.
Otras notas
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
L Eidels et al.
Proceedings of the National Academy of Sciences of the United States of America, 68(8), 1673-1677 (1971-08-01)
Genetic and biochemical evidence that sedoheptulose-7-phosphate is an obligatory precursor of the L-glycero-D-mannoheptose residues of the lipopolysaccharide of Salmonella was obtained by isolation and characterization of transketolase-negative mutants of Salmonella typhimurium. These mutants, which are defective in synthesis of sedoheptulose-7-phosphate
Sedoheptulose kinase regulates cellular carbohydrate metabolism by sedoheptulose 7-phosphate supply.
Csörsz Nagy et al.
Biochemical Society transactions, 41(2), 674-680 (2013-03-22)
Dynamic carbon re-routing between catabolic and anabolic metabolism is an essential element of cellular transformation associated with tumour formation and immune cell activation. Such bioenergetic adaptations are important for cellular function and therefore require tight control. Carbohydrate phosphorylation has been
John F Williams et al.
Photosynthesis research, 90(2), 125-148 (2006-12-13)
14C-Labelled octulose phosphates were formed during photosynthetic 14CO2 fixation and were measured in spinach leaves and chloroplasts. Because mono- and bisphosphates of D: -glycero- D: -ido-octulose are the active 8-carbon ketosugar intermediates of the L-type pentose pathway, it was proposed
Biosynthesis of 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone by yeasts.
Sasaki, M., et al.
Journal of Agricultural and Food Chemistry, 39, 934-938 (1991)
Mirjam M C Wamelink et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 823(1), 18-25 (2005-08-02)
We describe a liquid chromatography tandem mass spectrometry (LC-MS/MS) method to quantify pentose phosphate pathway intermediates (triose-3-phosphates, tetrose-4-phosphate, pentose-5-phosphate, pentulose-5-phosphates, hexose-6-phosphates and sedoheptulose-7-phosphate (sed-7P)) in bloodspots, fibroblasts and lymphoblasts. Liquid chromatography was performed using an ion pair loaded C(18) HPLC
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico