67563
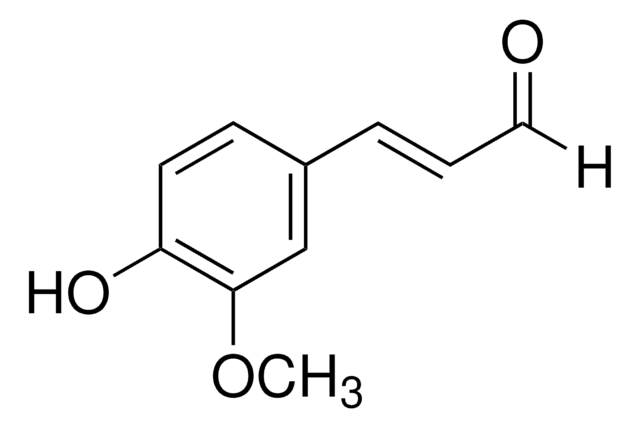
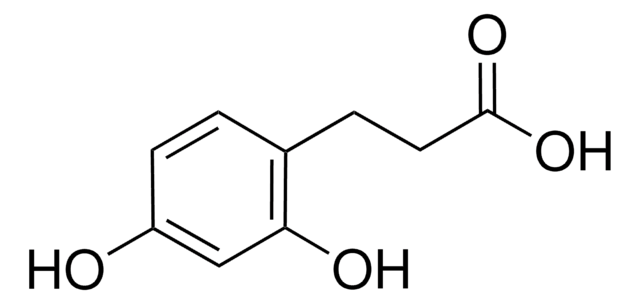
3,4-Dihydroxy-5-methoxycinnamic acid
≥95.0% (HPLC)
Sinónimos:
3-(3,4-Dihydroxy-5-methoxyphenyl)-2-propenoic acid, 3-Methoxycaffeic acid, 5-Hydroxyferulic acid
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥95.0% (HPLC)
Formulario
solid
aplicaciones
metabolomics
vitamins, nutraceuticals, and natural products
cadena SMILES
COc1cc(\C=C\C(O)=O)cc(O)c1O
InChI
1S/C10H10O5/c1-15-8-5-6(2-3-9(12)13)4-7(11)10(8)14/h2-5,11,14H,1H3,(H,12,13)/b3-2+
Clave InChI
YFXWTVLDSKSYLW-NSCUHMNNSA-N
Categorías relacionadas
Acciones bioquímicas o fisiológicas
Envase
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico