PHR1227
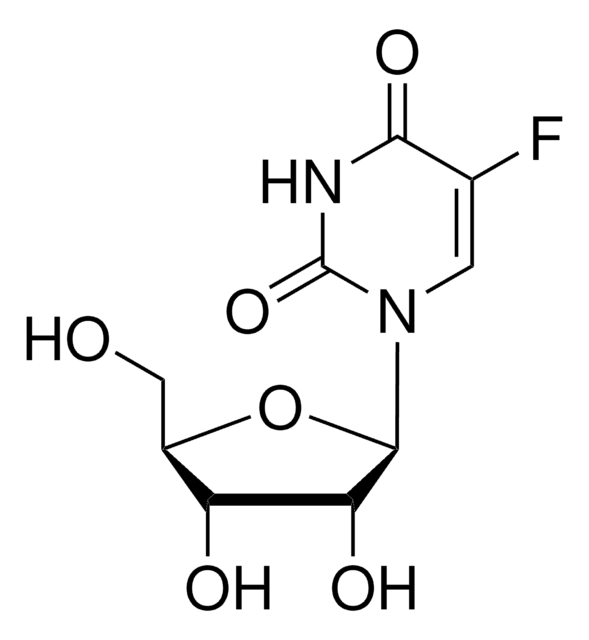
Fluorouracil
Pharmaceutical Secondary Standard; Certified Reference Material
Sinónimos:
5-Fluorouracil, 2,4-Dihydroxy-5-fluoropyrimidine, 5-FU, 5-Fluoro-2,4(1H,3H)-pyrimidinedione
About This Item
Productos recomendados
grado
certified reference material
pharmaceutical secondary standard
Nivel de calidad
Agency
traceable to BP 995
traceable to Ph. Eur. F0250000
traceable to USP 12790000
familia API
fluorouracil
CofA
current certificate can be downloaded
técnicas
HPLC: suitable
gas chromatography (GC): suitable
mp
282-286 °C (dec.) (lit.)
aplicaciones
cleaning products
cosmetics
food and beverages
personal care
pharmaceutical (small molecule)
Formato
neat
temp. de almacenamiento
2-30°C
cadena SMILES
FC1=CNC(=O)NC1=O
InChI
1S/C4H3FN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9)
Clave InChI
GHASVSINZRGABV-UHFFFAOYSA-N
Información sobre el gen
human ... TYMS(7298)
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Fluorouracil, an analog of the pyrimidines, belongs to the class of antimetabolite drugs. It is generally utilized for the remediation of tumors such as breast adenocarcinoma, gastrointestinal tract, ovary, head and neck.
Aplicación
Nota de análisis
Otras notas
Nota al pie de página
Productos recomendados
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Oral - Carc. 2
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico