86320
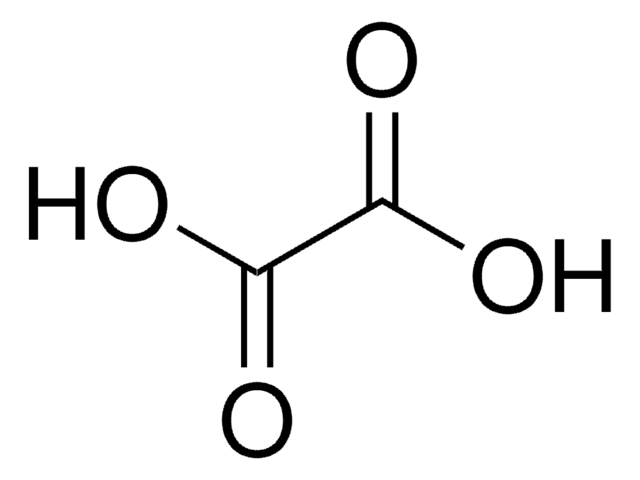
Tartronic acid
≥97.0%
Sinónimos:
Hydroxymalonic acid, Hydroxypropanedioic acid
About This Item
Productos recomendados
Ensayo
≥97.0%
Formulario
powder
mp
158-160 °C (dec.) (lit.)
grupo funcional
carboxylic acid
hydroxyl
temp. de almacenamiento
2-8°C
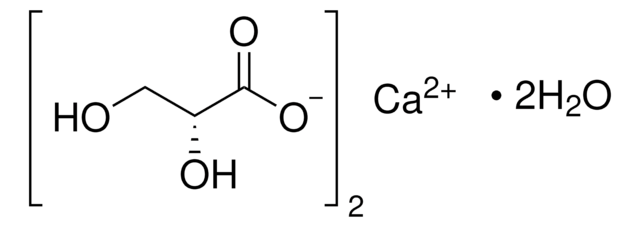
cadena SMILES
OC(C(O)=O)C(O)=O
InChI
1S/C3H4O5/c4-1(2(5)6)3(7)8/h1,4H,(H,5,6)(H,7,8)
Clave InChI
ROBFUDYVXSDBQM-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- Polymer synthesis for enhanced thermal conductivity: Tartronic acid is used to exploit enzyme reactions in polymer synthesis, significantly increasing the thermal conductivity of materials, which is pivotal in manufacturing and material science applications (Nan et al., 2023).
- Advances in green chemical treatments: This acid plays a role in the electro-oxidation pathways for treating glycerol waste, contributing to sustainable chemical processes and green chemistry applications, which are essential for reducing environmental impact (Cheng et al., 2021).
- Development in biodiesel by-products treatment: Tartronic acid is also involved in kinetic studies for the electrochemical conversion of glycerol, a by-product of biodiesel production, highlighting its role in renewable energy and waste valorization (Pérès et al., 2020).
- Base-free oxidation reactions: It aids in the development of base-free conditions for glycerol to glyceraldehyde oxidation reactions over platinum-based catalysts, offering advancements in catalysis and organic synthesis processes (Capron et al., 2019).
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico