Descripción general
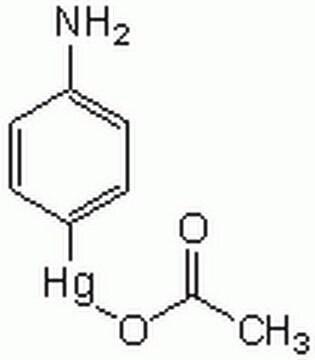
Recombinant, human pro-MMP-9 expressed in CHO cells. The calculated molecular weight is ~77 kDa, but the apparent molecular weight is ~92 kDa by SDS-PAGE. Useful for immunoblotting, substrate cleavage assays, and zymography. MMP-9 Proenzyme can be measured by its ability to degrade gelatin in a zymogram. 0.5 ng of enzyme is sufficient to visualize degraded gelatin with coomassie blue stain. Matrix metalloproteinases are members of a unique family of proteolytic enzymes that have a zinc ion at their active sites and can degrade collagens, elastin and other components of the extracellular matrix (ECM). These enzymes are present in normal healthy individuals and have been shown to have an important role in processes such as wound healing, pregnancy, and bone resorption. However, overexpression and activation of MMPs have been linked with a range of pathological processes and disease states involved in the breakdown and remodeling of the ECM. Such diseases include tumor invasion and metastasis, rheumatoid arthritis, periodontal disease and vascular processes such as angiogenesis, intimal hyperplasia, atherosclerosis and aneurysms. Recently, MMPs have been linked to neurodegenerative diseases such as Alzheimer’s, and amyotrophic lateral sclerosis (ALS). Natural inhibitors of MMPs, tissue inhibitor of matrix metalloproteinases (TIMPs) exist and synthetic inhibitors have been developed which offer hope of new treatment options for these diseases. Regulation of MMP activity can occur at the level of gene expression, including transcription and translation, level of activation, or at the level of inhibition by TIMPs. Thus, perturbations at any of these points can theoretically lead to alterations in ECM turnover. Expression is under tight control by pro- and anti-inflammatory cytokines and/or growth factors and, once produced the enzymes are usually secreted as inactive zymograms. Upon activation (removal of the inhibitory propeptide region of the molecules) MMPs are subject to control by locally produced TIMPs. All MMPs can be activated in vitro with organomercurial compounds (e.g., 4-aminophenylmercuric acetate), but the agents responsible for the physiological activation of all MMPs have not been clearly defined. Numerous studies indicate that members of the MMP family have the ability to activate one another. The activation of the MMPs in vivo is likely to be a critical step in terms of their biological behavior, because it is this activation that will tip the balance in favor of ECM degradation. The hallmark of diseases involving MMPs appear to be stoichiometric imbalance between active MMPs and TIMPs, leading to excessive tissue disruption and often degradation. Determination of the mechanisms that control this imbalance may open up some important therapeutic options of specific enzyme inhibitors.
Aplicación
Immunoblotting (1 µg protein/lane)
Substrate Cleavage Assay (1 µg protein/lane, see application references)
Zymography (1 µg protein/lane, see application references)
Envase
Please refer to vial label for lot-specific concentration.
Advertencia
Toxicity: Standard Handling (A)
Definición de unidad
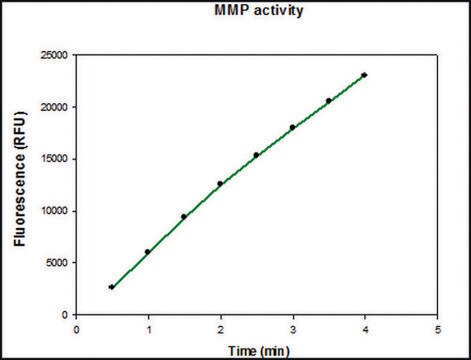
Specific activity is determined using 10 μM (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-Ala-Arg-NH₂ (excitation 320 nm, emission 405 nm), and 20 ng enzyme in 100 μl of 50 mM Tris-HCl, pH 7.5, 10 mM CaCl₂, 150 mM NaCl, and 0.05% BRIJ®-35 Detergent at room temperature.
Forma física
In 150 mM NaCl, 50 mM Tris-HCl, 10 mM CaCl₂, 0.05% BRIJ®-35 Detergent, pH 7.5.
Otras notas
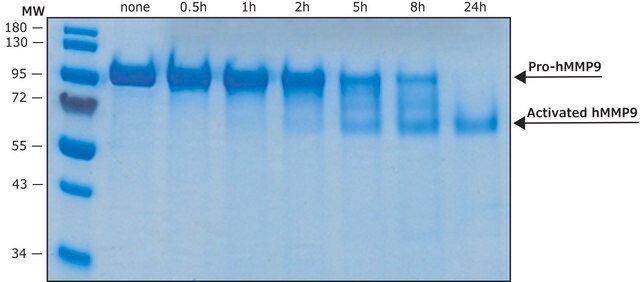
MMP-9 Proenzyme can be measured by its ability to degrade gelatin in a zymogram. 0.5 ng of enzyme is sufficient to visualize degraded gelatin with coomassie blue stain. The specific activity as measured with 10 µM (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-Ala-Arg-NH2 (excitation 320 nm, emission 405 nm), and 20 ng enzyme in 100 µl of 50 mM Tris-HCl, pH 7.5, 10 mM CaCl2, 150 mM NaCl, and 0.05% Brij-35 at room temperature, is >1,300 pmoles/min/µg. To activate MMP-9 proenzyme, prepare p-aminophenylmercuric acetate (APMA) concentrate in dimethylsulfoxide (DMSO). Add APMA to MMP-9 proenzyme to give a final APMA concentration of 1 mM. Incubate at 37°C for 16 to 24 h.
Parsons, S.L., et al. 1997. Br. J. Surg.84, 160.
Backstrom, J.R., et al. 1996. J. Neuro.16, 7910.
Lim, G.P., et al. 1996. J Neurochem.67, 251.
Sang, Q.X., et al. 1995. Biochim. Biophys. Acta.1251, 99.
Kenagy, R.D. and Clowes, A.W. 1994. in Inhibition of Matrix Metalloproteinases: Therapeutic Potential. Greenwald, R.A. and Golub L.M., Eds.: 462-465.
Zempo, N., et al. 1994. J. Vasc. Surg.20, 209.
Birkedal-Hansen, H. 1993. J. Periodontol.64, 474.
Stetler-Stevenson, W.G., et al. 1993. FASEB J.7, 1434.
Delaisse, J-M. and Vaes, G. 1992. in Biology and Physiology of the Osteoclast. B.R. Rifkin & C.V. Gay, Eds.: 290-314.
Jeffrey, J.J. 1992. in Wound Healing: Biochemical and Clinical Aspects. R.F. Diegelmann and W.J. Lindblad, Eds.: 177-194.
Jeffrey, J.J. 1991. Semin. Perinatol.15, 118.
Liotta, L.A., et al. 1991. Cell64, 327.
Harris, E. 1990. N. Engl. J. Med.322, 1277.
Información legal
Brij is a registered trademark of Croda International PLC
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany