MABS157
Anti-O-Linked N-Acetylglucosamine Antibody, clone RL2
clone RL2, from mouse
Sinónimos:
O-Linked N-Acetylglucosamine
About This Item
Productos recomendados
origen biológico
mouse
Nivel de calidad
forma del anticuerpo
purified immunoglobulin
tipo de anticuerpo
primary antibodies
clon
RL2, monoclonal
reactividad de especies (predicha por homología)
all
técnicas
affinity binding assay: suitable
electron microscopy: suitable
immunocytochemistry: suitable
immunoprecipitation (IP): suitable
western blot: suitable
isotipo
IgG1κ
Condiciones de envío
wet ice
modificación del objetivo postraduccional
unmodified
Información sobre el gen
human ... OGT(8473)
Descripción general
Especificidad
Inmunógeno
Aplicación
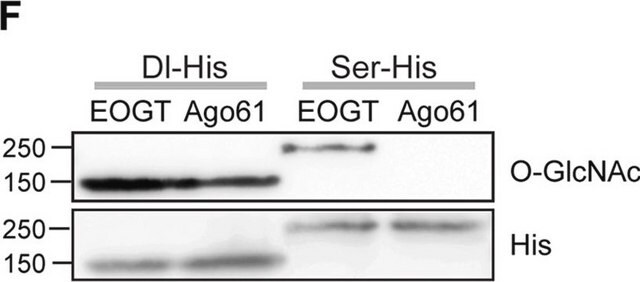
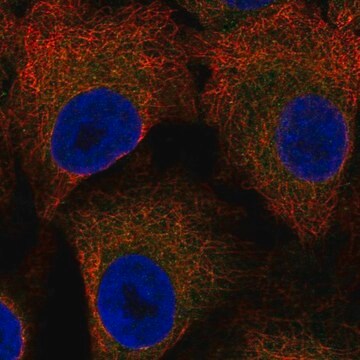
Immunocytochemistry Analysis: A representative lot immunostained nuclear envelopes, but not the nuclear interior, of digitonin-permeabilized HeLa cells. Clone RL2 stained the nuclear interior only among Triton X-100-permeabilized HeLa cells without intact nuclear envelopes (Adam, S.A., et al. (1990). J. Cell Biol. 111(3):807-816).
Affinity Binding Assay: A representative lot was radiolabeled with 125I and studied for its binding characteristics toward isolated rat liver nuclear envelopes (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-1156).
Electron Microscopy: A representative lot localized the O-GlcNAc immunoreactivity in isolated rat liver nuclear envelopes (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-1156).
Western Blotting Analysis: A representative lot detected O-GlcNAcylated proteins in rat liver nuclear envelopes preparations (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-1156; Holt, G.D., et al. (1987). J. Cell Biol. 104(5):1157-1164).
Immunoprecipitation Analysis: A representative lot immunoprecipitated O-GlcNAcylated proteins from solubilized rat liver nuclear envelopes preparations. Pretreatment of nuclear envelopes preparations with galactosyltrarnsferase prevented the immunoprecipitation of glycoproteins by clone RL2 (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-1156; Holt, G.D., et al. (1987). J. Cell Biol. 104(5):1157-1164).
Calidad
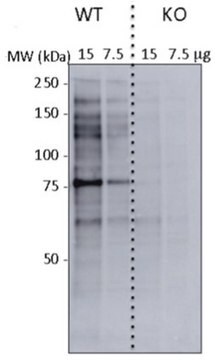
Western Blotting Analysis: 1.0 µg/mL of this antibody detected O-Linked N-Acetylglucosamine in 10 µg of HeLa cell lysate.
Descripción de destino
Forma física
Otras notas
¿No encuentra el producto adecuado?
Pruebe nuestro Herramienta de selección de productos.
Código de clase de almacenamiento
12 - Non Combustible Liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico