681675
Wortmannin
≥98% (HPLC), solid, PI3-kinase inhibitor, Calbiochem
Sinónimos:
Wortmannin, KY 12420, MLCK Inhibitor II
About This Item
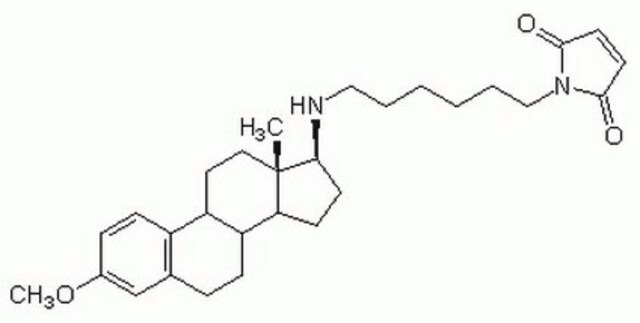
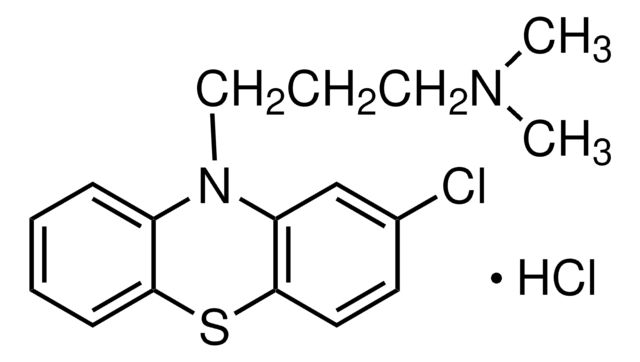
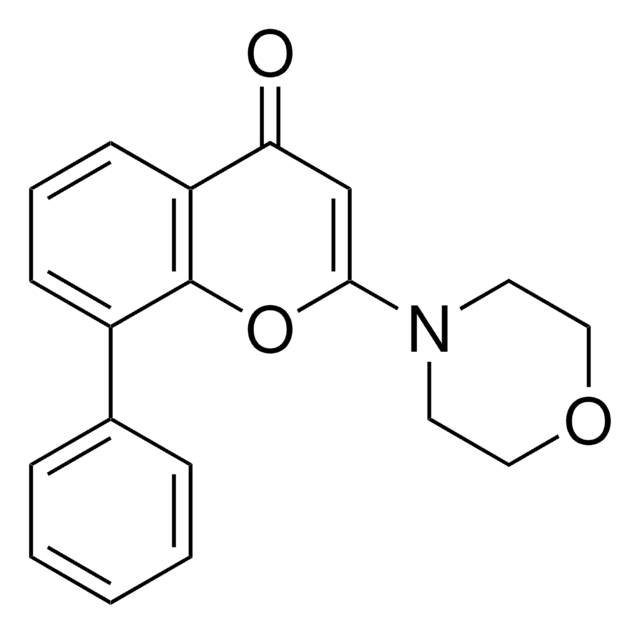
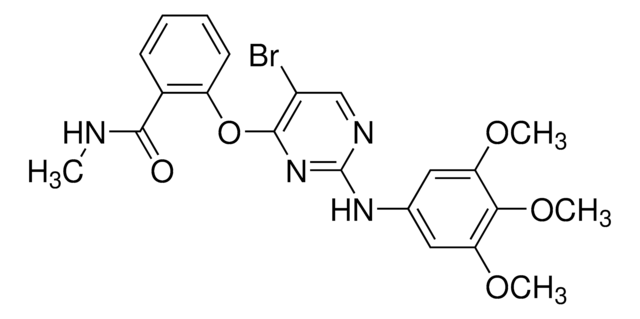
Productos recomendados
Nombre del producto
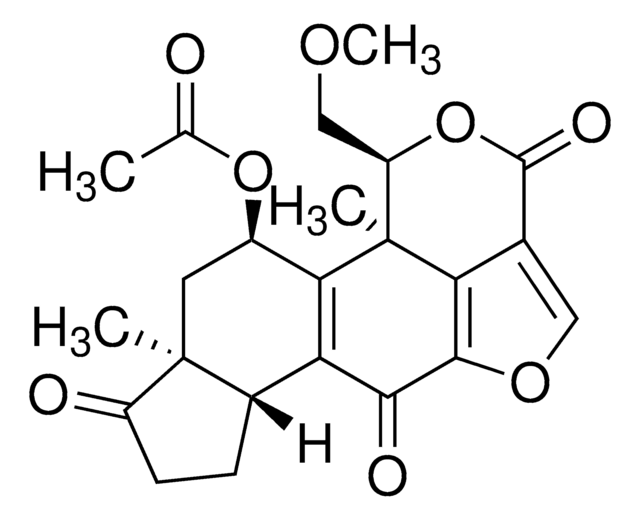
Wortmannin, Wortmannin, CAS 19545-26-7, is a cell-permeable, potent, selective, and irreversible inhibitor of PI3-Kinase (IC₅₀ = 5 nM). Does not affect any upstream signaling events.
Nivel de calidad
Ensayo
≥98% (HPLC)
Formulario
solid
fabricante / nombre comercial
Calbiochem®
condiciones de almacenamiento
OK to freeze
protect from light
color
white to off-white
solubilidad
DMSO: 25 mg/mL
Condiciones de envío
ambient
temp. de almacenamiento
−20°C
InChI
1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1
Clave InChI
QDLHCMPXEPAAMD-QAIWCSMKSA-N
Descripción general
Acciones bioquímicas o fisiológicas
phosphatidylinositol-3-kinase
Envase
Advertencia
Reconstitución
Otras notas
Nakamura, I., et al. 1995. FEBS Lett. 361, 79.
Ferby, I.M., et al. 1994. J. Biol. Chem. 269, 30485.
Okada, T., et al. 1994. J. Biol. Chem. 269, 3568.
Wymann, M.P. and Arcaro, A. 1994. Biochem. J.298, 517.
Arcaro, A. and Wymann, M.P. 1993. Biochem. J.296, 297.
Nakanishi, S., et al. 1992. J. Biol. Chem. 267, 2157.
Bonser, R.W., et al. 1991. Br. J. Pharmacol.103, 1237.
Información legal
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 1 Inhalation - Acute Tox. 1 Oral - Acute Tox. 2 Dermal
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico