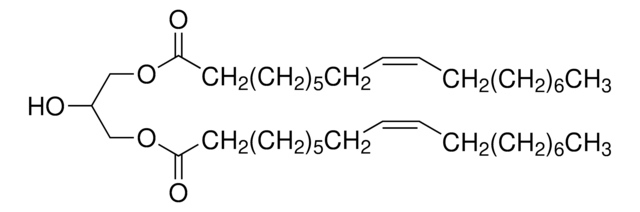800819C
Avanti
18:0-22:6 DG
1-stearoyl-2-docosahexaenoyl-sn-glycerol, chloroform
Sinónimos:
1-octadecanoyl-2-(4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl)-sn-glycerol; DG(18:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0)
About This Item
Productos recomendados
Ensayo
>99% (TLC)
Formulario
liquid
envase
pkg of 1 × 5 mL (800819C-10mg)
fabricante / nombre comercial
Avanti Research™ - A Croda Brand 800819C
concentración
2 mg/mL (800819C-10mg)
tipo de lípido
neutral lipids
neutral glycerides
Condiciones de envío
dry ice
temp. de almacenamiento
−20°C
Categorías relacionadas
Descripción general
Diacylglycerol mimicks the effects of the tumor-promoting compounds phorbol esters.
Aplicación
- in the preparation of Golgi-like liposomes
- to study its effect on conventional protein kinase C (cPKC) and novel protein kinase C (nPKC) isozymes in vitro
- as a substrate for the measurement of diacylglycerol kinase η1 (DGKη1) activity in vitro
Envase
Almacenamiento y estabilidad
Otras notas
Dry samples of diacylglycerol in chloroform, using a stream of nitrogen. Dissolve the residue in an appropriate volume of ethanol or DMSO, then dilute to the desired aqueous medium.
Most biological responses saturate at 20 to 250 μM sn-1,2-dioctanoylglycerol. Only sn-1,2 isomers appear to be active.
Información legal
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Órganos de actuación
Central nervous system, Liver,Kidney
Clase de riesgo para el agua (WGK)
WGK 3
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico