V3700
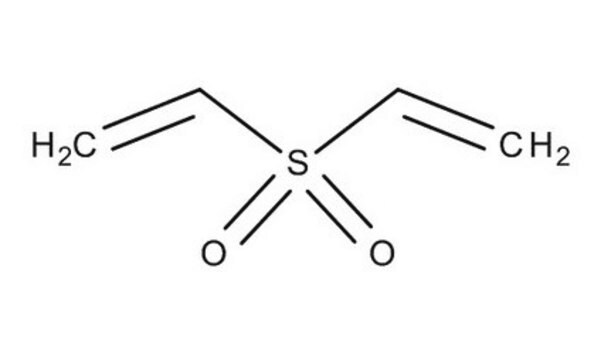
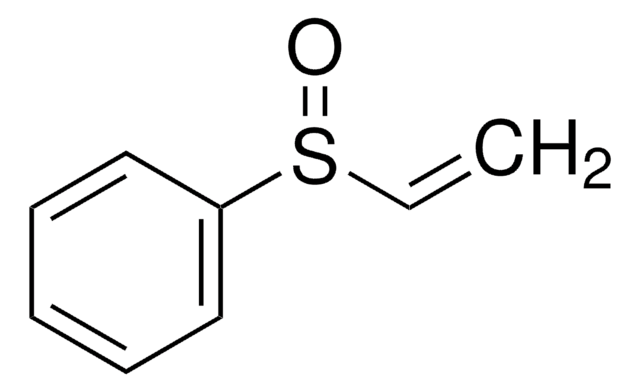
Divinyl sulfone
contains hydroquinone as inhibitor, ≥96%
Sinónimos:
Vinyl sulfone
About This Item
Productos recomendados
Nivel de calidad
Análisis
≥96%
contiene
hydroquinone as inhibitor
índice de refracción
n20/D 1.476 (lit.)
bp
234 °C (lit.)
mp
−26 °C (lit.)
densidad
1.177 g/mL at 25 °C (lit.)
temp. de almacenamiento
2-8°C
cadena SMILES
C=CS(=O)(=O)C=C
InChI
1S/C4H6O2S/c1-3-7(5,6)4-2/h3-4H,1-2H2
Clave InChI
AFOSIXZFDONLBT-UHFFFAOYSA-N
Información sobre el gen
human ... LOC129293(129293)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Aplicación
- A cross-linking agent to synthesize divinyl sulfone-crosslinked hyaluronic acid hydrogels for specific biomedical applications, such as tissue engineering or drug delivery.
- A cross-linking agent to develop the conducting polymer film with MXene layers. This crosslinking can enhance the mechanical properties and stability of the composite film.
DVS and its mono and di-substituted derivatives are useful starting materials in the preparation of thiomorpholine 1,1-dioxides and other synthetically important macro- and the heterocycles.
DVS may be used to shrink proofing cotton by crosslinking it with cellulose.
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 1 Dermal - Acute Tox. 2 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
215.6 °F - closed cup
Punto de inflamabilidad (°C)
102 °C - closed cup
Equipo de protección personal
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico