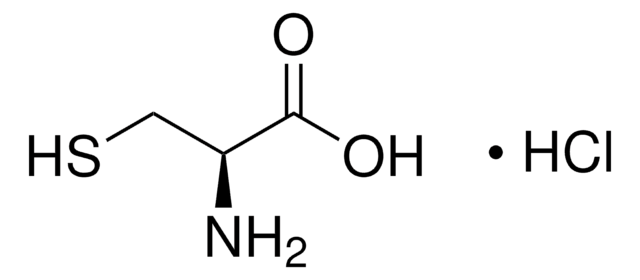
P6526
Iron(III) pyrophosphate
soluble crystals
Sinónimos:
Diphosphoric acid iron(III) salt, Ferric pyrophosphate
About This Item
Productos recomendados
grado
for analytical purposes
Nivel de calidad
Formulario
soluble crystals
composición
Iron content, 10.5-12.5%
concentración
10.5-12.5% (titration)
aplicaciones
battery manufacturing
cadena SMILES
[Fe+3].[Fe+3].[Fe+3].[Fe+3].[O-]P([O-])(=O)OP([O-])([O-])=O.[O-]P([O-])(=O)OP([O-])([O-])=O.[O-]P([O-])(=O)OP([O-])([O-])=O
InChI
1S/4Fe.3H4O7P2/c;;;;3*1-8(2,3)7-9(4,5)6/h;;;;3*(H2,1,2,3)(H2,4,5,6)/q4*+3;;;/p-12
Clave InChI
CADNYOZXMIKYPR-UHFFFAOYSA-B
Categorías relacionadas
Descripción general
Aplicación
- As a cathode material for rechargeable batteries such as Na-ion batteries.
- To fabricate a hybrid overlayer on the surface of metal oxides to boost photo electrochemical solar water splitting reaction.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico