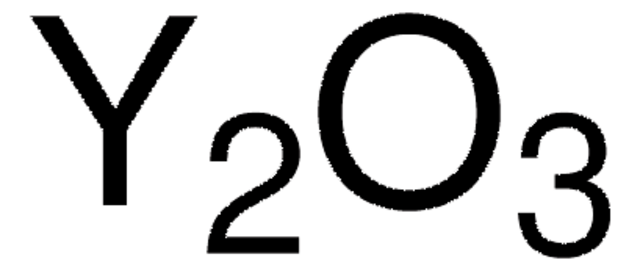L4000
Lanthanum(III) oxide
≥99.9%
Sinónimos:
Lanthana, Lanthanum sesquioxide, Lanthanum trioxide
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥99.9%
Formulario
powder
idoneidad de la reacción
reagent type: catalyst
core: lanthanum
densidad
6.51 g/mL at 25 °C (lit.)
aplicaciones
battery manufacturing
cadena SMILES
O=[La]O[La]=O
InChI
1S/2La.3O
Clave InChI
KTUFCUMIWABKDW-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
It can be used to prepare thermal-barrier coatings with a high thermal expansion coefficient and low thermal conductivity.
It can also be used as a recyclable catalytic system for the synthesis of diphenyl sulfides and selenides.
Características y beneficios
- High refractive index
- Thermal stability
- Hardness
- High dielectric constant
Código de clase de almacenamiento
13 - Non Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Innovation in dental restorative materials is driven by the need for biocompatible and natural-appearing restoration alternatives. Conventional dental materials like amalgam and composite resins have inherent disadvantages.
Rechargeable solid-state batteries are becoming increasingly important due to wide-spread use in computers, portable electronics, and vehicular applications.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico