907723
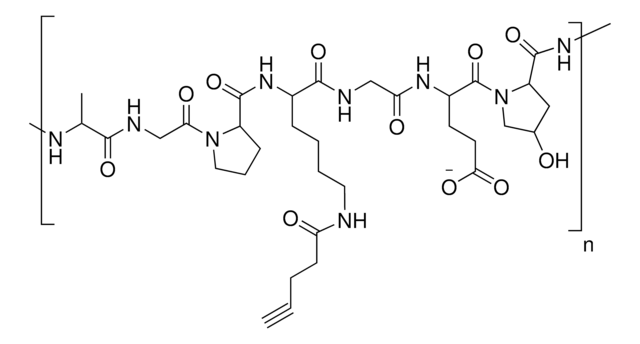
Azide functionalized gelatin
degree of substitution >80%
Sinónimos:
Azide functionlized gelatin, Azide-modified gelatin, Clickable gelatin
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Código UNSPSC:
12352125
NACRES:
NA.23
Productos recomendados
descripción
Degree of substitution: greater than 80% by TNBS method
NMR: Conforms to structure
Formulario
powder
color
white to pale yellow
temp. de almacenamiento
−20°C
Categorías relacionadas
Descripción general
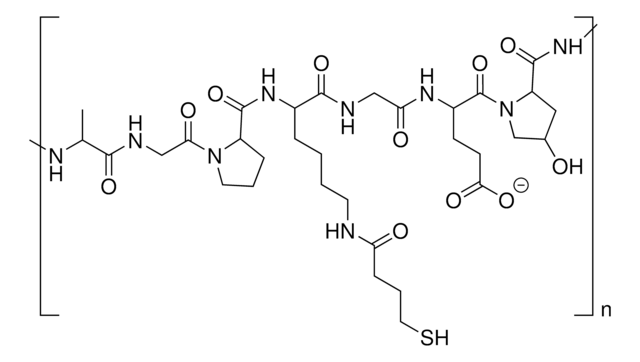
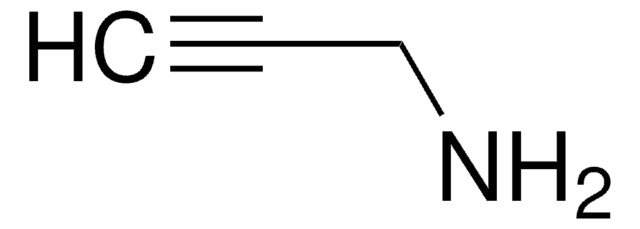
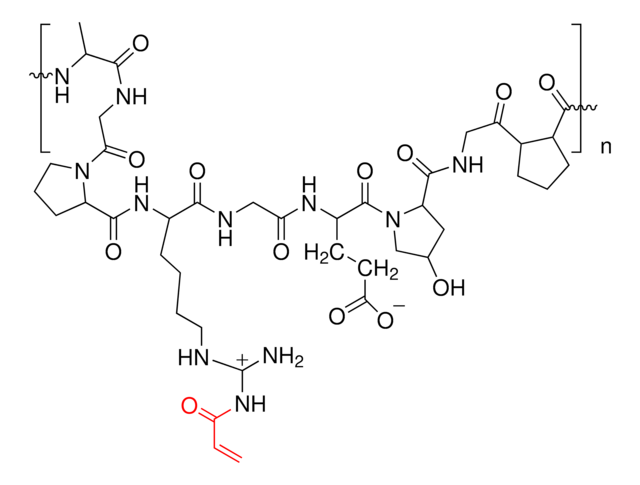
Due to its biodegradablity and biocompatibility, gelatin is routinely used in hydrogels for biomedical applications such as drug delivery, tissue engineering, and 3D bioprinting. Gelatin-based hydrogels are synthesized by the crosslinking of functionalized gelatins. Depending on the identity of the functional groups, several different processes can be used to synthesize crosslinked gelatin hydrogels, including radical-based (either thermal or photochemical) and click chemistry methods. Azide-functionalized gelatin can be used in the synthesis of hydrogels using click chemistry with alkyne-containing substrates.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Gelatin hydrogels via thiol-ene chemistry
Russo L, et al.
Monatshefte fur Chemie / Chemical Monthly, 147, 587-592 (2016)
Vinh X Truong et al.
Biomacromolecules, 16(7), 2246-2253 (2015-06-10)
In this study, we present a method for the fabrication of in situ forming gelatin and poly(ethylene glycol)-based hydrogels utilizing bioorthogonal, strain-promoted alkyne-azide cycloaddition as the cross-linking reaction. By incorporating nitrobenzyl moieties within the network structure, these hydrogels can be
Thiol-yne `click?/coupling chemistry and recent applications in polymer and materials synthesis and modification.
Andrew B. Lowe
Polymer, 55 (2014)
Sandeep T Koshy et al.
Advanced healthcare materials, 5(5), 541-547 (2016-01-26)
Injectable gelatin hydrogels formed with bioorthogonal click chemistry (ClickGel) are cell-responsive ECM mimics for in vitro and in vivo biomaterials applications. Gelatin polymers with pendant norbornene (GelN) or tetrazine (GelT) groups can quickly and spontaneously crosslink upon mixing, allowing for
Masato Tamura et al.
Scientific reports, 5, 15060-15060 (2015-10-10)
This paper describes the generation of "click-crosslinkable" and "photodegaradable" gelatin hydrogels from the reaction between dibenzocycloctyl-terminated photoclevable tetra-arm polyethylene glycol and azide-modified gelatin. The hydrogels were formed in 30 min through the click-crosslinking reaction. The micropatterned features in the hydrogels were
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico