900856
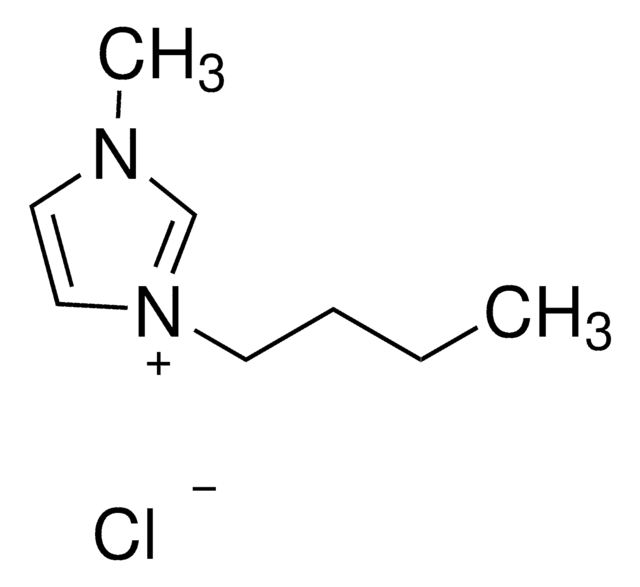
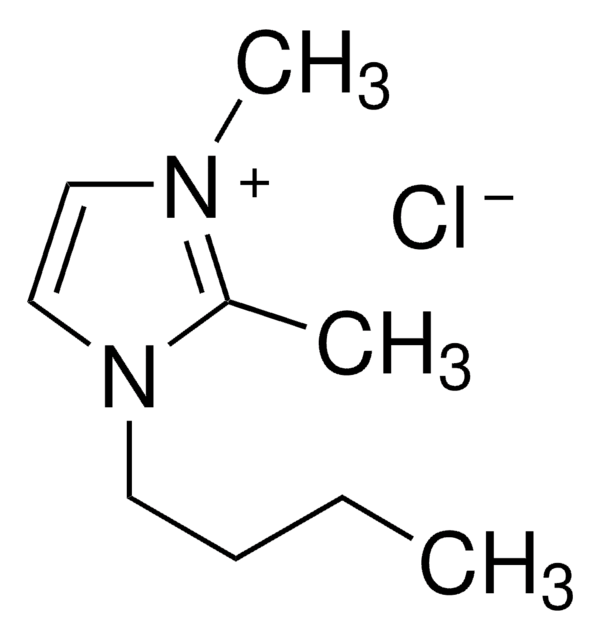
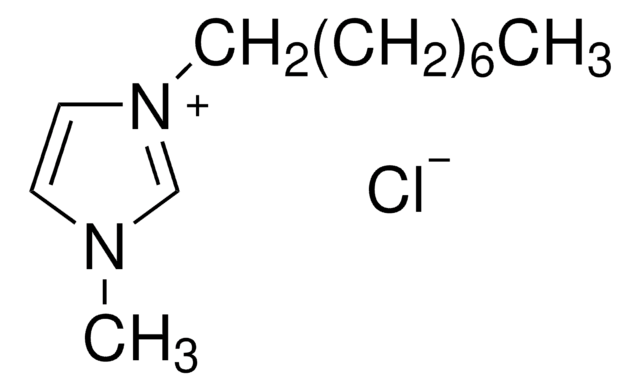
1-Butyl-3-methylimidazolium chloride
≥99%
Sinónimos:
1-Methyl-3-butylimidazolium chloride, N-Butyl-N′-methylimidazolium chloride, BMIMCl
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥99%
Formulario
solid
características de los productos alternativos más sostenibles
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
~70 °C
aplicaciones
battery manufacturing
categoría alternativa más sostenible
, Aligned
cadena SMILES
[Cl-].CCCCn1cc[n+](C)c1
InChI
1S/C8H15N2.ClH/c1-3-4-5-10-7-6-9(2)8-10;/h6-8H,3-5H2,1-2H3;1H/q+1;/p-1
Clave InChI
FHDQNOXQSTVAIC-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Click here for more information.
Aplicación
Precaución
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
377.6 °F - (External MSDS)
Punto de inflamabilidad (°C)
192 °C - (External MSDS)
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Dr. Sun reviews the recent advances in solid-state rechargeable batteries and cover the fundamentals of solid electrolytes in solid-state batteries, the theory of ion conduction, and the structures and electrochemical processes of solid-state Li batteries.
Ionic Liquids Based Electrolytes for Rechargeable Batteries
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico