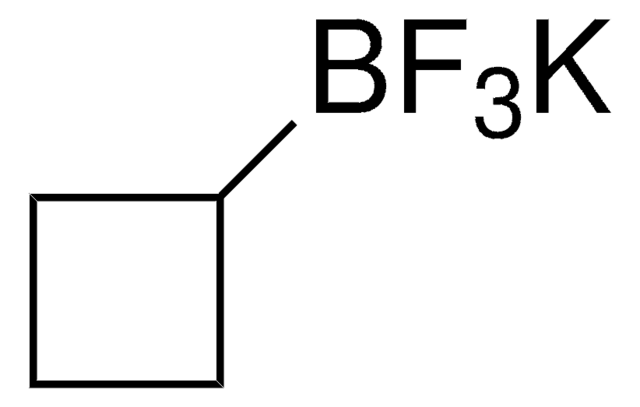
747157
Potassium pentafluroroethyltrifluoroborate
95%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C2BF8K
Número de CAS:
Peso molecular:
225.92
Número MDL:
Código UNSPSC:
12352103
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Análisis
95%
formulario
solid
mp
252-257 °C
temp. de almacenamiento
2-8°C
cadena SMILES
F[B-](F)(F)C(F)(F)C(F)(F)F.[K+]
InChI
1S/C2BF8.K/c4-1(5,2(6,7)8)3(9,10)11;/q-1;+1
Clave InChI
PSJPJAFBTMLFFX-UHFFFAOYSA-N
Aplicación
Organotrifluoroborate involved in:
Organotrifluoroborates as versatile and stable boronic acid surrogates
- Suzuki Miyaura cross-coupling reactions, and polymerization reactions
- Synthesis of photonic crystals
- Synthesis of sensitizers for dye-sensitized solar cells
- Mannich / diastereoselective hydroamination reaction sequence
Organotrifluoroborates as versatile and stable boronic acid surrogates
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Gary A Molander et al.
Journal of the American Chemical Society, 130(47), 15792-15793 (2008-11-05)
A method was developed for the hydroboration of alkenyl-containing organotrifluoroborates to generate dibora intermediates. The reactivity differences between organotrifluoroborates and trialkylboranes facilitated the cross-coupling of the borane moiety of these intermediates in a highly chemoselective fashion with aryl halides, leaving
Roberto Grisorio et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(27), 8054-8061 (2010-06-04)
The mechanism of the Suzuki-Heck (SuHe) polymerisation of 2,7-dibromo-9,9-di(n-dodecyl)fluorene (1) with potassium vinyl trifluoroborate (PVTB) for the synthesis of poly(fluorenylene vinylene)s (PFVs) has been investigated. In the first stage, a palladium-catalysed chain-growth AA/B(C)-type polycondensation occurs, as evidenced by the linear
Emilio Alacid et al.
The Journal of organic chemistry, 74(6), 2321-2327 (2009-02-17)
Potassium vinyl and alkenyltrifluoroborates are cross-coupled with aryl and heteroaryl bromides using 1 mol % Pd loading of 4-hydroxyacetophenone oxime derived palladacycle or Pd(OAc)2 as precatalysts, K2CO3 as base, and TBAB as additive and water reflux under conventional or microwave
S. Achelle;
Journal of Polymer Science: Part A, General Papers, 48, 2659-2665 (2010)
Jenny M Baxter Vu et al.
Organic letters, 13(15), 4056-4059 (2011-07-14)
A new two-step synthesis of highly substituted pyrrolidines has been developed. Chiral silane Lewis acid promoted enantioselective Mannich reactions of silyl ketene imines with acylhydrazones may be used to access bishomoallylic benzoic hydrazides that in turn may be cyclized to
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![Phenyl[3-(trifluoromethyl)phenyl]iodonium triflate ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/424/062/057593f4-e032-4d3e-bed6-6be37c1ae76d/640/057593f4-e032-4d3e-bed6-6be37c1ae76d.png)