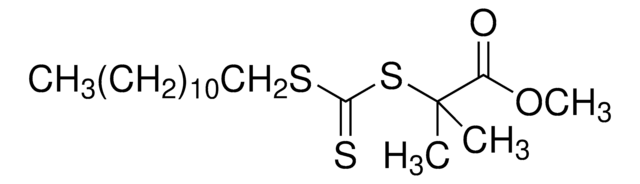
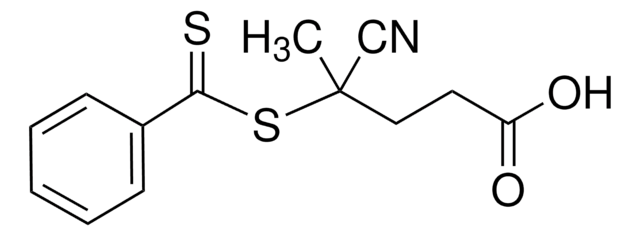
723274
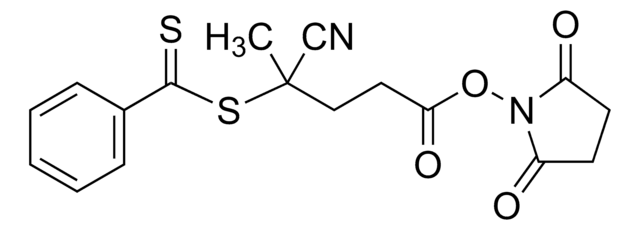
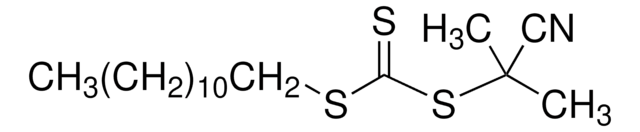
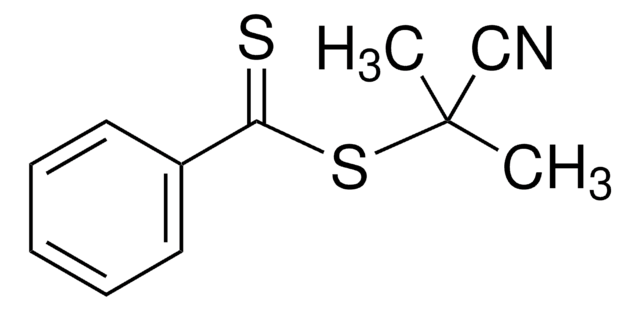
4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid
97% (HPLC)
Sinónimos:
4-Cyano-4-(((dodecylthio)carbonothioyl)thio)pentanoic acid
About This Item
Productos recomendados
Análisis
97% (HPLC)
formulario
solid
mp
64-68 °C
temp. de almacenamiento
−20°C
cadena SMILES
CCCCCCCCCCCCSC(=S)SC(C)(CCC(O)=O)C#N
InChI
1S/C19H33NO2S3/c1-3-4-5-6-7-8-9-10-11-12-15-24-18(23)25-19(2,16-20)14-13-17(21)22/h3-15H2,1-2H3,(H,21,22)
Clave InChI
RNTXYZIABJIFKQ-UHFFFAOYSA-N
Descripción general
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The supply of low cost, high purity and effective Reversible addition−fragmentation chain-transfer (RAFT) Agents is the essential element in the industrial implementation of RAFT polymerization technology.
A series of polymerization were carried out using RAFT agents and monomers yielding well-defined polymers with narrow molecular weight distributions. The process allows radical-initiated growing polymer chains to degeneratively transfer reactivity from one to another through the use of key functional groups (dithioesters, trithiocarbonates, xanthates and dithiocarbamates). RAFT agents help to minimize out-of-control growth and prevent unwanted termination events from occurring, effectively controlling polymer properties like molecular weight and polydispersity. RAFT agents are commercially available. RAFT does not use any cytotoxic heavy metal components (unlike ATRP).
RAFT (Reversible Addition Fragmentation chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
The modification of biomacromolecules, such as peptides and proteins, through the attachment of synthetic polymers has led to a new family of highly advanced biomaterials with enhanced properties.
Protocolos
RAFT (Reversible Addition-Fragmentation chain Transfer) is a form of living radical polymerization involving conventional free radical polymerization of a substituted monomer in the presence of a suitable chain transfer (RAFT) reagent.
Sigma-Aldrich presents an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanol](/deepweb/assets/sigmaaldrich/product/structures/839/520/64c23004-f340-460f-a379-8670a35d0433/640/64c23004-f340-460f-a379-8670a35d0433.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)