672068
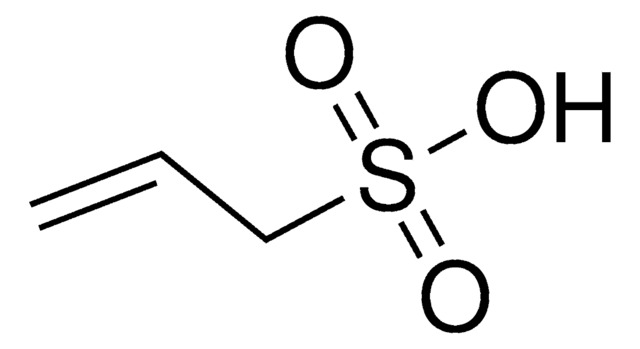
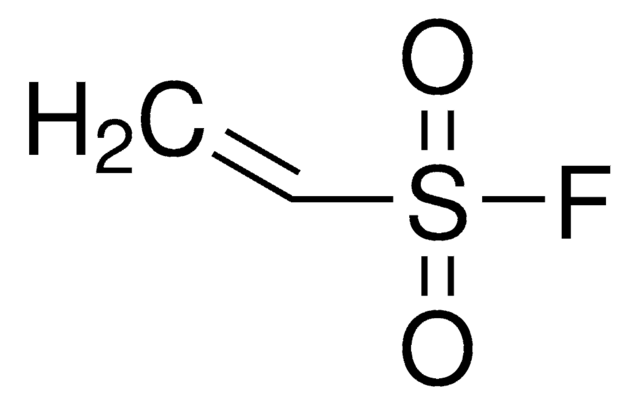
Vinylphosphonic acid
≥90% (T)
Sinónimos:
Ethenephosphonic acid, Ethylenephosphonic acid, P-Ethenylphosphonic acid
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥90% (T)
impurezas
≤7.0% water
mp
36 °C (Lit. dry VPA) (lit.)
densidad
1.37 g/mL at 20 °C (lit.)
cadena SMILES
OP(O)(=O)C=C
InChI
1S/C2H5O3P/c1-2-6(3,4)5/h2H,1H2,(H2,3,4,5)
Clave InChI
ZTWTYVWXUKTLCP-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
It can also be used as an organic building block to prepare (E)-styryl phosphonic acid derivatives by reacting with various aryl halides via Pd-catalyzed Heck coupling reaction.
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Met. Corr. 1 - Skin Corr. 1B
Código de clase de almacenamiento
8A - Combustible corrosive hazardous materials
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
467.6 °F
Punto de inflamabilidad (°C)
242 °C
Equipo de protección personal
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![Bis[2-(methacryloyloxy)ethyl] phosphate](/deepweb/assets/sigmaaldrich/product/structures/128/336/4e7a3e38-338c-423e-95b8-70d9d1f8e121/640/4e7a3e38-338c-423e-95b8-70d9d1f8e121.png)