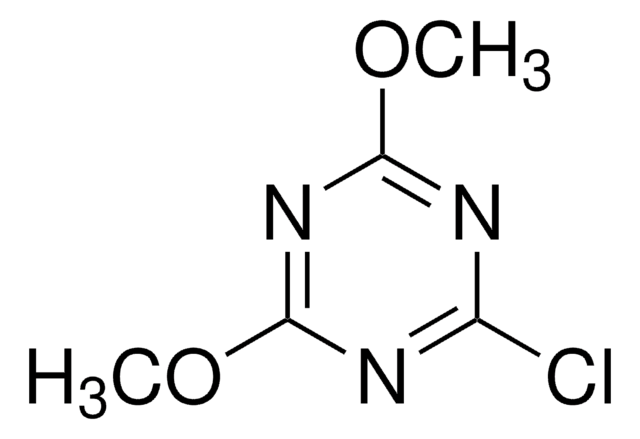
529249
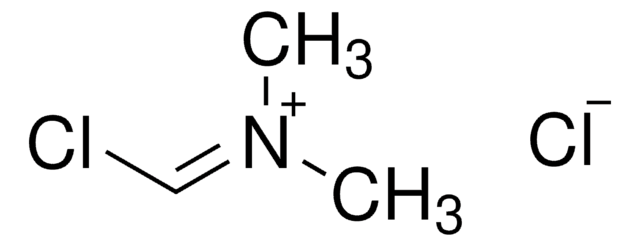
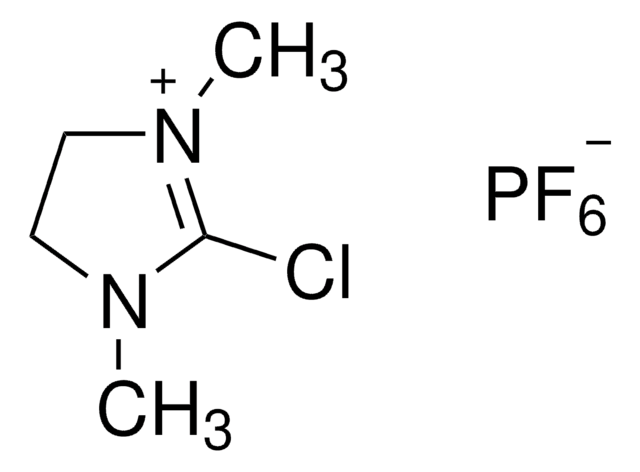
2-Chloro-1,3-dimethylimidazolinium chloride
for peptide synthesis
Sinónimos:
2-Chloro-4,5-dihydro-1,3-dimethyl-1H-imidazolium chloride, DMC
About This Item
Productos recomendados
product name
2-Chloro-1,3-dimethylimidazolinium chloride,
formulario
crystalline
Nivel de calidad
idoneidad de la reacción
reaction type: Coupling Reactions
mp
133-140 °C (lit.)
aplicaciones
peptide synthesis
grupo funcional
chloro
cadena SMILES
[Cl-].CN1CC[N+](C)=C1Cl
InChI
1S/C5H10ClN2.ClH/c1-7-3-4-8(2)5(7)6;/h3-4H2,1-2H3;1H/q+1;/p-1
Clave InChI
AEBBXVHGVADBHA-UHFFFAOYSA-M
Aplicación
Tagged glucose as an intermediate in the synthesis of branched oligosaccharides
Fluorescent chemosensors
1,2-Diamines as inhibitors of co-activator associated arginine methyltransferase 1
Allosteric glucokinase activators
Reactant for synthesis of:
Organic azides from primary amines
Reagent for aza-Henry reactions
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
N-Acylimidazoles were recognized in the early 1950s as reactive intermediates suitable for the acylation of amino compounds. The search for better coupling reagents than DCC led to the development of CDI (1,1’-carbonyldiimidazole) and related carbonylimidazoles.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico