517135
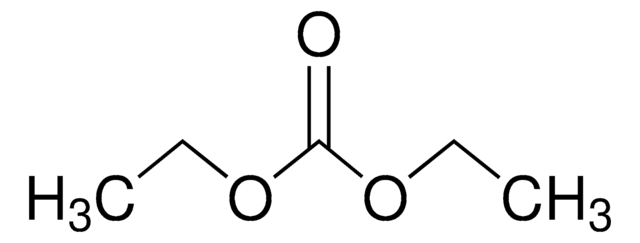
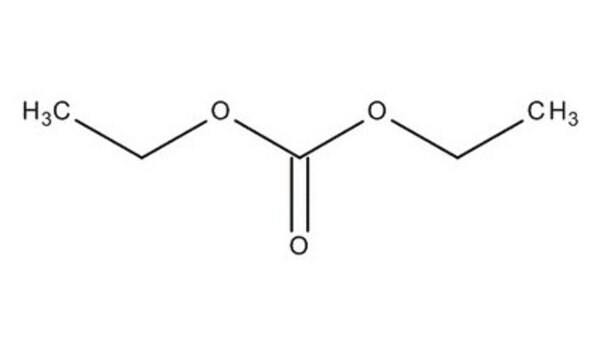
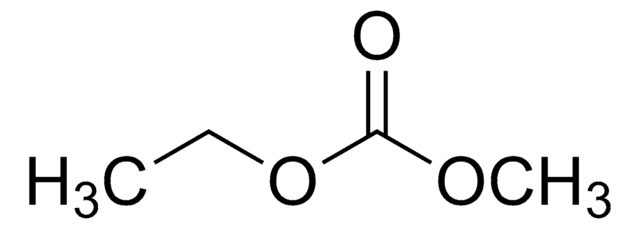
Diethyl carbonate
anhydrous, ≥99%
Sinónimos:
Diatol, Eufin, H-DEC
About This Item
Productos recomendados
grado
anhydrous
Nivel de calidad
densidad de vapor
4.1 (vs air)
presión de vapor
10 mmHg ( 23.8 °C)
59 mmHg ( 37.8 °C)
Análisis
≥99%
características de los productos alternativos más sostenibles
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurezas
<0.002% water
<0.005% water (100 mL)
índice de refracción
n20/D 1.384 (lit.)
bp
126-128 °C (lit.)
mp
−43 °C (lit.)
solubilidad
water: insoluble
densidad
0.975 g/mL at 25 °C (lit.)
grupo funcional
carbonate
categoría alternativa más sostenible
cadena SMILES
O=C(OCC)OCC
InChI
1S/C5H10O3/c1-3-7-5(6)8-4-2/h3-4H2,1-2H3
Clave InChI
OIFBSDVPJOWBCH-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- Synthesis of β-enamino esters.
- Synthesis of carbamates and unsymmetrical alkyl carbonates, via reaction with aliphatic amines or alcohols by using a hybrid organic-inorganic material prepared by anchoring TBD to MCM-41 silica.
- As solvent in ruthenium catalyzed direct functionalisation of arene C-H bonds by aryl halides.
- To compose the commercial liquid electrolyte for lithium ion batteries.
- Homogeneous alkoxycarbonylation of cellulose.
Características y beneficios
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Flam. Liq. 3
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
77.0 °F - closed cup
Punto de inflamabilidad (°C)
25 °C - closed cup
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Dr. Schmuch, Dr. Siozios, Professor Dr. Winter, and Dr. Placke review the challenges and opportunities of nickelrich layered oxide cathode materials. They discuss production processes for the layered oxide cathode materials as well as their chemistry and morphology.
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico