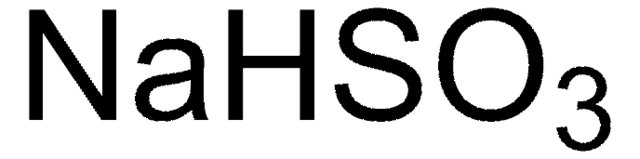
455849
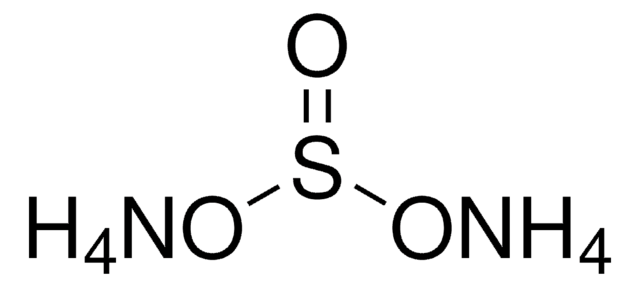
Ammonium hydrogensulfate
99.99% trace metals basis
Sinónimos:
Ammonium bisulfate, Ammonium sulfate monobasic
About This Item
Productos recomendados
Análisis
99.99% trace metals basis
formulario
crystalline
impurezas
≤200 mg/kg Trace metallic impurities analysis (ICP)
bp
350 °C (dec.)(lit.)
mp
121-145 °C (lit.)
densidad
1.79 g/mL at 25 °C (lit.)
cadena SMILES
N.OS(O)(=O)=O
InChI
1S/H3N.H2O4S/c;1-5(2,3)4/h1H3;(H2,1,2,3,4)
Clave InChI
BIGPRXCJEDHCLP-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- Enhancement of Aesthetic Dental CAD-CAM Materials through Surface Etching with a Mixed Aqueous Solution of Ammonium Fluoride and Ammonium Hydrogen Sulfate - This study explores the potential of ammonium hydrogen sulfate in surface etching applications for dental materials, focusing on its low toxicity and effective etching capabilities (Y Nishizawa et al., 2024).
- Thermodynamics of ammonioalunite precipitation in ammonium aluminum sulfate solution - Investigates the thermodynamic properties of ammonium aluminum sulfate solutions, providing insights into chemical processes involving ammonium hydrogen sulfate (X Yang et al., 2020).
- Hygroscopic behavior and chemical composition evolution of internally mixed aerosols composed of oxalic acid and ammonium sulfate - Studies the hygroscopic properties of mixed aerosol particles, including those formed with ammonium hydrogen sulfate, to understand atmospheric chemical processes better (X Wang et al., 2017).
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Dam. 1 - Skin Corr. 1B
Código de clase de almacenamiento
8B - Non-combustible corrosive hazardous materials
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico