429074
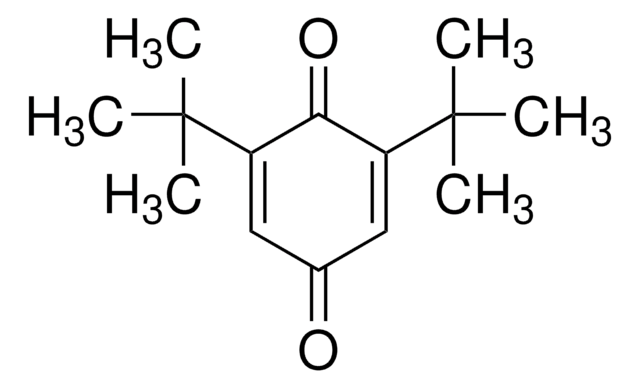
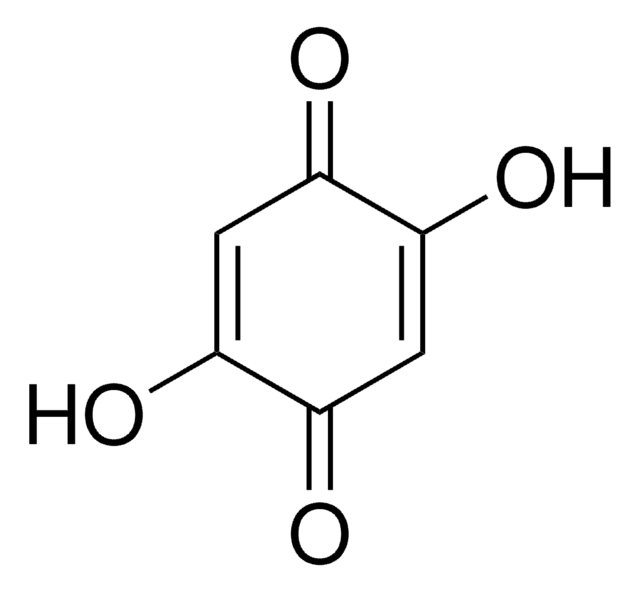
2-tert-Butyl-1,4-benzoquinone
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
(CH3)3CC6H3(=O)2
Número de CAS:
Peso molecular:
164.20
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
98%
Formulario
solid
mp
54-58 °C (lit.)
grupo funcional
ketone
cadena SMILES
CC(C)(C)C1=CC(=O)C=CC1=O
InChI
1S/C10H12O2/c1-10(2,3)8-6-7(11)4-5-9(8)12/h4-6H,1-3H3
Clave InChI
NCCTVAJNFXYWTM-UHFFFAOYSA-N
Descripción general
2-tert-Butyl-1,4-benzoquinone (TBQ, TBBQ, tBQ, BuBQ, BQ , tert-butyl-p-quinone) is a 1,4-benzoquinone derivative. It is a major metabolite of the food additive, butylated hydroxyanisole (BHA). TBQ is reported to be strongly cytotoxic in human monocytic leukemia U937 cells. TBQ is an oxidation product of 2-tert-butylhydroquinone (TBHQ). Studies confirm that TBQ induces apoptosis and cell proliferation inhibition in chronic myelogenous leukemia (CML) cells. Its binding interactions with lysozyme has been examined and found to be intermediate between BHA and TBHQ. It has been reported to be synthesized by the titanium superoxide catalyzed oxidation of 2-tert-butylphenol using aq. 30% H2O2. TBQ is one of the main neoformed compounds from TBHQ decomposition in PLA-TBHQ film (Poly lactic acid).
Aplicación
2-tert-Butyl-1,4-benzoquinone may be used in the synthesis of azatrioxa[8]circulene.
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
2-tert-butyl-1, 4-benzoquinone Induces Apoptosis in Chronic Myeloid Leukemia Cells Resistant to Imatinib via Inducing Caspase-Dependent Bcr-Abl Downregulation.
Shi X, et al.
Medicinal Chemistry, 4, 784-790 (2014)
R Kahl et al.
Toxicology, 59(2), 179-194 (1989-12-01)
The synthetic antioxidant butylated hydroxyanisole (BHA) stimulates superoxide formation in rat liver microsomes up to 10-fold. This stimulation is prevented by the monooxygenase inhibitor metyrapone and does not occur when NADH is consumed instead of NADPH indicating that metabolic activation
W H Kalus et al.
Environmental health perspectives, 102(1), 96-99 (1994-01-01)
We examined t-butylhydroquinone (t-BHQ) and t-butylquinone (t-BuQ), two of the major microsomal metabolites of the synthetic antioxidant butylated hydroxyanisole (BHA), for their ability to react with the xenobiotic arylamines aniline and N-methylaniline. A number of substances were isolated by thin-layer
Azatrioxa [8] circulenes: Planar Anti-Aromatic Cyclooctatetraenes.
Nielsen CB, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 19(12), 3898-3904 (2013)
P A Schilderman et al.
Carcinogenesis, 16(3), 507-512 (1995-03-01)
The food additive butylated hydroxyanisole (BHA) has been shown to induce gastrointestinal hyperplasia in rodents by an unknown mechanism. The relevance of this observation for human risk assessment is not clear. We therefore analysed the effect of BHA and its
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico