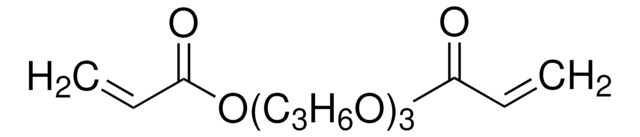411744
1,4-Butanediol diacrylate
technical grade, contains ~75 ppm hydroquinone as inhibitor
Sinónimos:
1,4-Bis(acryloyloxy)butane, Tetramethylene diacrylate
About This Item
Productos recomendados
grado
technical grade
Nivel de calidad
Análisis
87%
formulario
liquid
contiene
~75 ppm hydroquinone as inhibitor
índice de refracción
n20/D 1.456 (lit.)
bp
83 °C/0.3 mmHg (lit.)
densidad
1.051 g/mL at 25 °C (lit.)
temp. de almacenamiento
2-8°C
cadena SMILES
C=CC(=O)OCCCCOC(=O)C=C
InChI
1S/C10H14O4/c1-3-9(11)13-7-5-6-8-14-10(12)4-2/h3-4H,1-2,5-8H2
Clave InChI
JHWGFJBTMHEZME-UHFFFAOYSA-N
Descripción general
Aplicación
- As a precursor to synthesize joint-linker hydrogels with good mechanical strength and used as scaffold materials in bone tissue engineering as biomimetics for natural tissues and also in drug delivery systems.
- To prepare anti-fouling coating for dental composites.
- As a crosslinking agent to prepare hydrophobic acrylic intraocular lens(IOL) materials with reduced glistening.
- As a precursor to fabricate poly(β-amino ester) based solid polymer electrolytefilms for Li-ion batteries. BDDA enhances the ionic conductivity of theelectrolyte films.
Palabra de señalización
Danger
Frases de peligro
Clasificaciones de peligro
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1A
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
>235.4 °F
Punto de inflamabilidad (°C)
> 113 °C
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Photo-Crosslinkable Gelatin Hydrogel: Versatile Materials for (High Resolution) Additive Manufacturing
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico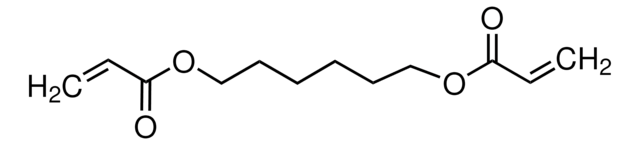
![1,8-Diazabiciclo[5.4.0]undec-7-eno 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)