37760
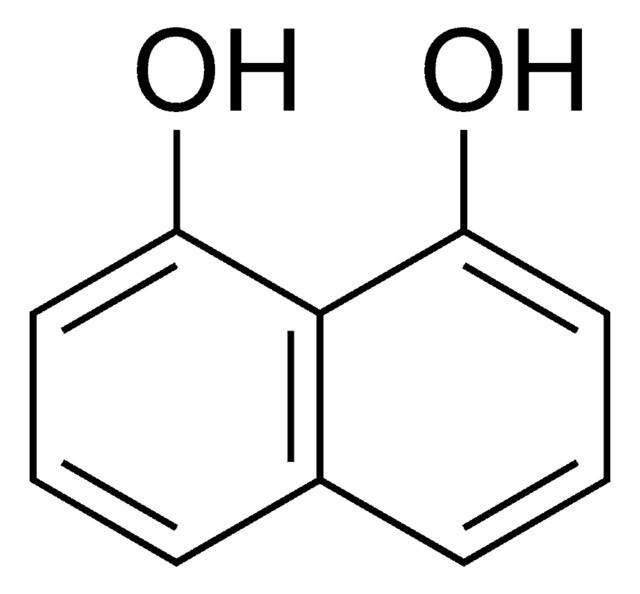
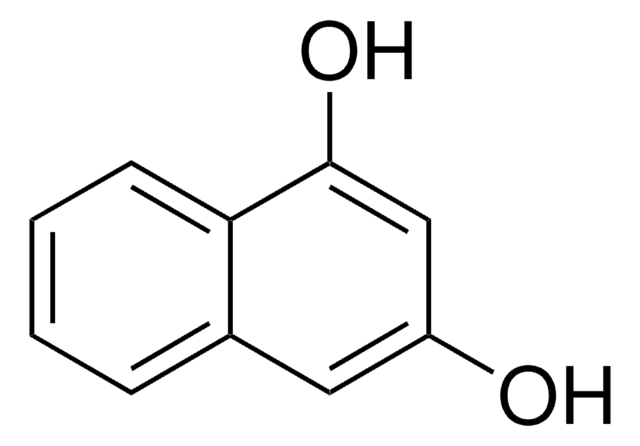
2,3-Dihydroxynaphthalene
≥98.0% (HPLC)
Sinónimos:
2,3-Naphthalenediol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C10H6(OH)2
Número de CAS:
Peso molecular:
160.17
Beilstein:
742375
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
≥98.0% (HPLC)
residuo de sublimación
≤1%
mp
161-165 °C (lit.)
162-164 °C
cadena SMILES
Oc1cc2ccccc2cc1O
InChI
1S/C10H8O2/c11-9-5-7-3-1-2-4-8(7)6-10(9)12/h1-6,11-12H
Clave InChI
JRNGUTKWMSBIBF-UHFFFAOYSA-N
Información sobre el gen
human ... BAD(572)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
2,3-Dihydroxynaphthalene is a polyhydroxy phenol. It is an aromatic dihydroxy compound having hydroxyl groups at ortho positions. Its reaction with molybdenum(VI) complexes has been reported. The asymmetric oxidative coupling polymerization of 2,3-dihydroxynaphthalene using the Cu(I)-bisoxazoline complex as catalyst has been reported to afford poly(2,3-dihydroxy-1,4-naphthylene), having a continuous 1,1′-bi-2-naphthol main chain structure. The nitrodisplacement reaction between nitrophthalodinitriles and 2,3-dihydroxynaphthalene has been investigated.
Aplicación
2,3-Dihydroxynaphthalene may be used in the following studies:
- Construction of dinaphtho[2,1-b;2′,3′-d]furan-6-ol, via dehydration reaction in the presence of strong acid.
- As fused ring catecholate type ligand for the surface modification of nanocrystalline TiO2 particles.
- As adsorptive and competing ligand during the chemical speciation of iron in seawater by cathodic stripping voltammetry.
- Synthesis of cyclotriphosphazene derivatives, used as non-halogen flame retardants
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral - Eye Dam. 1
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
347.0 °F
Punto de inflamabilidad (°C)
175 °C
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Application of cyclophosphazene derivatives as flame retardants for ABS.
Shin YJ, et al.
Journal of Industrial and Engineering Chemistry (Amsterdam, Netherlands), 16(3), 364-367 (2010)
Chemical speciation of iron in seawater by cathodic stripping voltammetry with dihydroxynaphthalene.
Constant M G van den Berg
Analytical chemistry, 78(1), 156-163 (2005-12-31)
The chemical speciation of iron in seawater is determined by cathodic stripping voltammetry using 2,3-dihydroxynaphthalene (DHN) as adsorptive and competing ligand. The optimized conditions include a DHN concentration of 0.5-1 microM, seawater at its original pH of 8, and equilibration
Kentaro Nakanishi et al.
The Journal of organic chemistry, 79(6), 2625-2631 (2014-02-26)
The construction of dinaphtho[2,1-b;2',3'-d]furan-6-ol was developed via a dehydration reaction involving two molecules of 2,3-dihydroxynaphthalene in the presence of a strong acid. Starting from the dinaphthofuran, a variety of butterfly shaped derivatives were synthesized. The optical properties of these compounds
Tatjana D Savić et al.
Nanoscale, 4(5), 1612-1619 (2012-02-09)
Surface modification of nanocrystalline TiO(2) particles (45 Å) with catecholate-type ligands consisting of an extended aromatic ring system, i.e., 2,3-dihydroxynaphthalene and anthrarobin, was found to alter the optical properties of the nanoparticles in a similar way to modification with catechol.
Synthesis of bis (ether anhydride) s for poly (ether imide) s having 1, 2-linked units by nitrodisplacement with catechol derivatives.
Eastmond GC and Paprotny J.
Macromolecules, 28(7), 2140-2146 (1995)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico