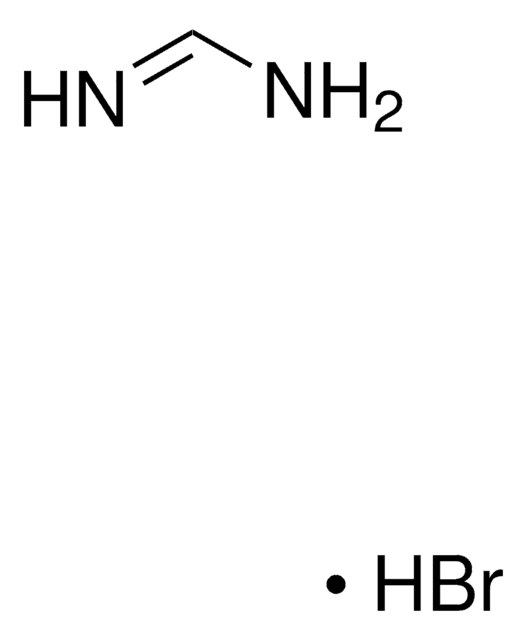
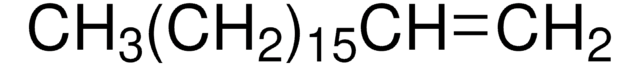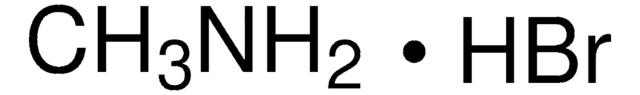211141
Lead(II) bromide
≥98%
Sinónimos:
Dibromolead, Lead dibromide
About This Item
Productos recomendados
Nivel de calidad
Análisis
≥98%
formulario
powder
idoneidad de la reacción
reagent type: catalyst
core: lead
bp
892 °C (lit.)
mp
371 °C (lit.)
solubilidad
ethanol: insoluble(lit.)
densidad
6.66 g/mL at 25 °C (lit.)
cadena SMILES
Br[PbH2]Br
InChI
1S/2BrH.Pb/h2*1H;/q;;+2/p-2
Clave InChI
ZASWJUOMEGBQCQ-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 1A - STOT RE 2
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The past several decades have seen major advancements in the synthesis of metal nanomaterials. Most recently, controlled synthesis has become versatile enough to regulate the exact number of atoms and ligands of very small metal nanoparticles, referred to as “clusters”.
Next generation solar cells have the potential to achieve conversion efficiencies beyond the Shockley-Queisser (S-Q) limit while also significantly lowering production costs.
Dr. Perini and Professor Correa-Baena discuss the latest research and effort to obtain higher performance and stability of perovskite materials.
For several decades, the need for an environmentally sustainable and commercially viable source of energy has driven extensive research aimed at achieving high efficiency power generation systems that can be manufactured at low cost.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico