ALD00598
CITU
≥95%
Synonym(s):
1,1,3,3-Tetramethyl-2-(4,5,6,7-tetrachloro-1,3-dioxoisoindolin-2-yl)isouronium
hexafluorophosphate(V)
About This Item
Recommended Products
Quality Level
Assay
≥95%
form
powder
reaction suitability
reaction type: Coupling Reactions
SMILES string
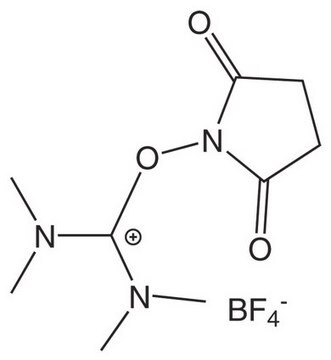
F[P-](F)(F)(F)(F)F.Clc1c2c(c(c(c1Cl)Cl)Cl)C(=O)N(C2=O)OC(=[N+](C)C)N(C)C
InChI
1S/C13H12Cl4N3O3.F6P/c1-18(2)13(19(3)4)23-20-11(21)5-6(12(20)22)8(15)10(17)9(16)7(5)14;1-7(2,3,4,5)6/h1-4H3;/q+1;-1
InChI key
AKPKDOSZCGGWNT-UHFFFAOYSA-N
General description
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Novabiochem® offers a large number of coupling reagents for in situ activation. In situ activating reagents are easy to use, fast reacting – even with sterically hindered amino acids, and their use is generally free of side reactions.
Related Content
The Baran Group works with Sigma-Aldrich in providing a portfolio of zinc-based reagents promoting difluoromethylation, trifluoromethylation, trifluoroethylation and isopropylation of aryl and heteroaryl motifs. Baran’s lab has also helped introduce a portable desaturase (Tz0Cl), which promotes the installation of alcohol and amine groups and leaves behind a highly useful tosyl group for further transformations.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| ALD00598-25G | 4061833491492 |
| ALD00598-5G | 4061833341896 |
| ALD00598-100G | 4061833315453 |
| ALD00598-1G | 4061824807332 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![COMU 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate Novabiochem®](/deepweb/assets/sigmaaldrich/product/images/237/337/13566c06-8931-4cc2-8621-c8742a392cd6/640/13566c06-8931-4cc2-8621-c8742a392cd6.jpg)
