445460
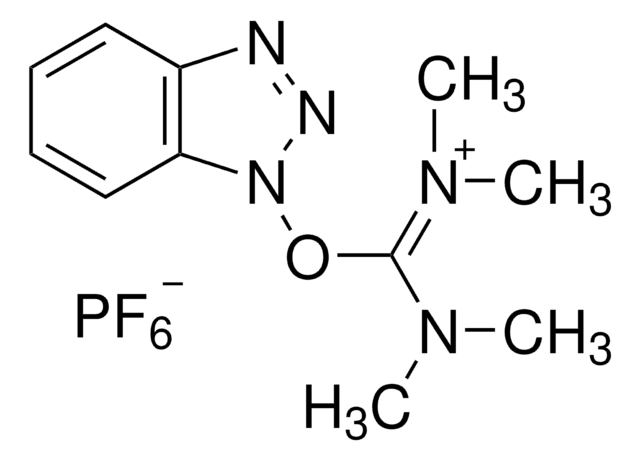
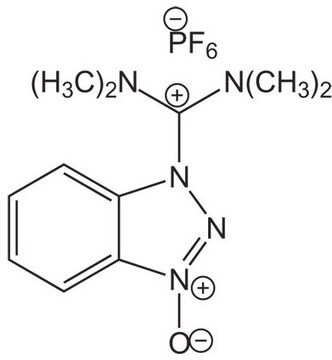
HATU
97%, for peptide synthesis
Synonym(s):
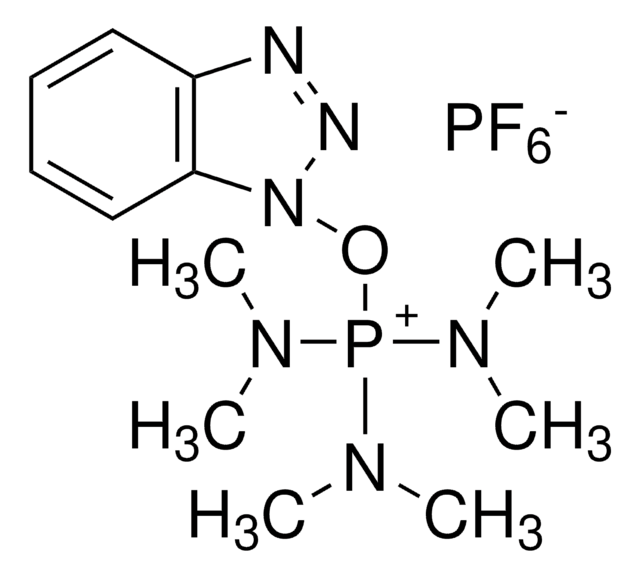
1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate, N-[(Dimethylamino)-1H-1,2,3-triazolo-[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide
About This Item
Recommended Products
product name
HATU, 97%
Quality Level
Assay
97%
form
solid
reaction suitability
reaction type: Coupling Reactions
mp
183-185 °C (lit.)
application(s)
peptide synthesis
functional group
amine
storage temp.
2-8°C
SMILES string
F[P-](F)(F)(F)(F)F.CN(C)[C+](N(C)C)n1n[n+]([O-])c2ncccc12
InChI
1S/C10H15N6O.F6P/c1-13(2)10(14(3)4)15-8-6-5-7-11-9(8)16(17)12-15;1-7(2,3,4,5)6/h5-7H,1-4H3;/q+1;-1
InChI key
FKBFHOSFPRWJNV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Synthesis of Aurora A kinase inhibitors
HPLC assay to determine D- and L- acid enantiomers in human plasma
Amide bond formation reactions
Catalyst for:
Selective acylation
Selecocyclization-oxidation deselenation sequence
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1A
Supplementary Hazards
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The special need of SPPS for rapid and highly efficient coupling reagents led to the development of several new reagents starting from BOP (Benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate).
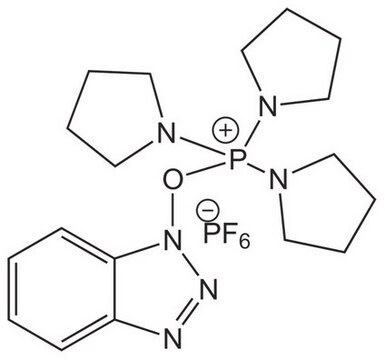
COMU is a non-explosive coupling agent suitable for solution phase & solid phase peptide synthesis. Its activity meets or exceeds that of HATU and its water-soluble by-product are easily removed.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service