909394
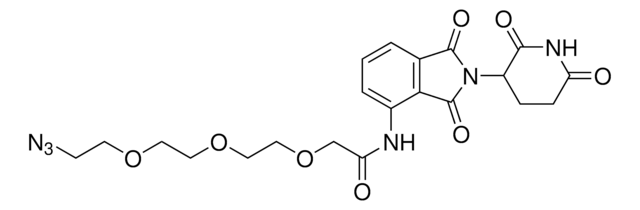
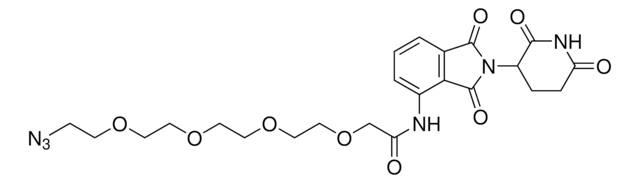
Pomalidomide-PEG5-azide
≥95%
Synonym(s):
17-Azido-N-(2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)-3,6,9,12,15-pentaoxaheptadecanamide, Crosslinker−E3 Ligase ligand conjugate, Pomalidomide conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
Recommended Products
ligand
pomalidomide
Assay
≥95%
form
powder
reaction suitability
reaction type: click chemistry
reagent type: ligand-linker conjugate
functional group
azide
storage temp.
2-8°C
SMILES string
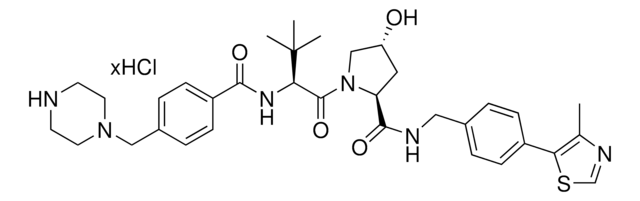
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NC(COCCOCCOCCOCCOCCN=[N+]=[N-])=O)=O)NC1=O
Application
Automate your CRBN-PEG based PROTACs with Synple Automated Synthesis Platform (SYNPLE-SC002)
Other Notes
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Legal Information
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service