All Photos(3)
About This Item
Empirical Formula (Hill Notation):
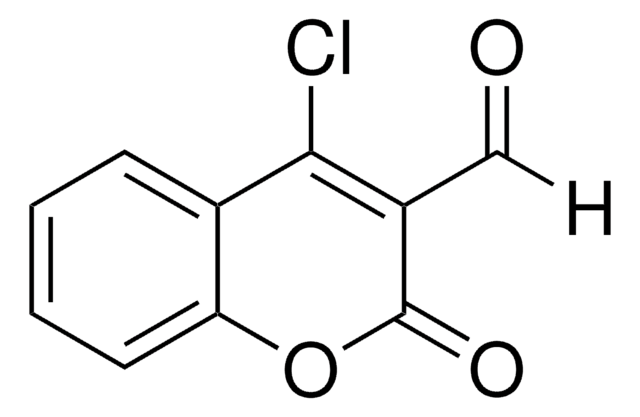
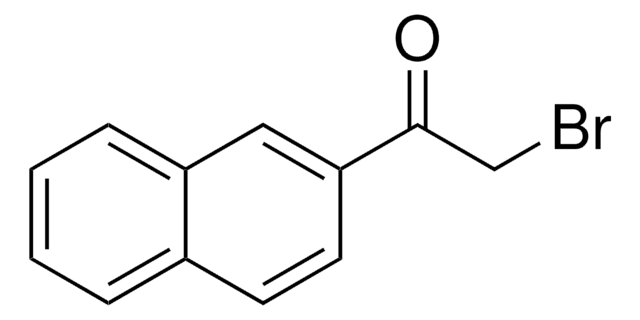
C11H7BrO3
CAS Number:
Molecular Weight:
267.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
164-168 °C (lit.)
functional group
bromo
ester
ketone
SMILES string
BrCC(=O)C1=Cc2ccccc2OC1=O
InChI
1S/C11H7BrO3/c12-6-9(13)8-5-7-3-1-2-4-10(7)15-11(8)14/h1-5H,6H2
InChI key
NTYOLVNSXVYRTJ-UHFFFAOYSA-N
General description
3-(Bromoacetyl)coumarin can be synthesized via the bromination of 3-acetylcoumarin in chloroform.
Application
3-(bromoacetyl)coumarin may be used in the synthesis of the following:
- 3-(bromoacetyl)coumarin oxime via reaction with hydroxylamine hydrochloride in methanol
- 3-(bromoacetyl)coumarin-O-methyloxime via reaction with O-benzylhydroxylammonium chloride/diluted HCl in methanol
- 3-(bromoacetyl)coumarin-O-benzyloxime via reaction with O-benzyl hydroxylamine hydrochloride in methanol
- 3-[2′-(2′′-arylidenehydrazinyl)thiazolyl]coumarins via reaction with benzaldehyde thiosemicarbazones
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
"Synthesis and Antibacterial Activity of Quinolone-Based Compounds Containing a Coumarin Moiety"
Emami S, et al.
Arch. Pharm. (Weinheim), 341(01), 42-48 (2008)
"Synthesis and Oral Hypoglycemic Activity of 3-[5'-Methyl-2'-aryl-3'-(thiazol-2"-yl amino) thiazolidin-4'-one] coumarin Derivatives"
Kini D and Ghate M
Journal of Chemistry, 8(01), 386- 390 (2011)
Saman Khan et al.
Chemico-biological interactions, 290, 64-76 (2018-05-29)
Coumarin is an important bioactive pharmacophore. It is found in plants as a secondary metabolite and exhibits diverse pharmacological properties including anticancer effects against different malignancies. Therapeutic efficacy of coumarin derivatives depends on the pattern of substitution and conjugation with
Ewa Poboży et al.
Mikrochimica acta, 172(3-4), 409-417 (2011-04-08)
Perfluorinated carboxylic acids (PFCAs) represent an important group of persistent perfluorinated organic compounds commonly determined in environmental and biological samples. A reversed-phase HPLC method was developed based on derivatization of the PFCAs with the commercially available fluorescent reagent 3-bromoacetyl coumarin.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service