C85603
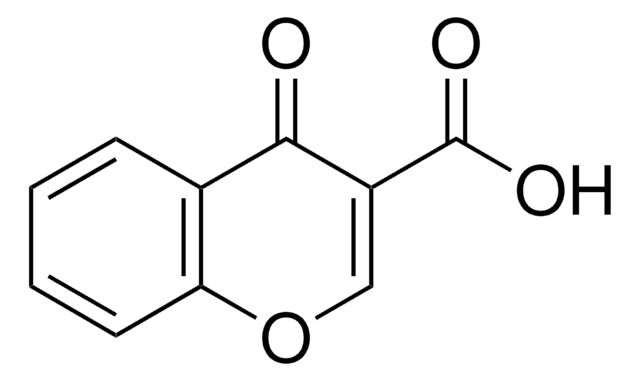
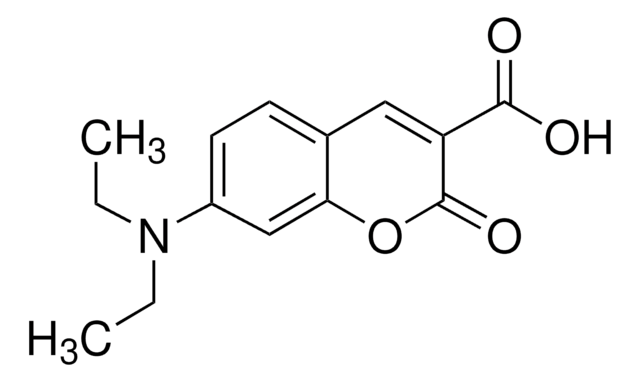
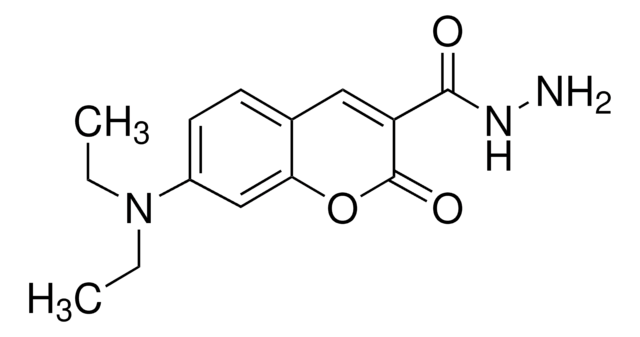
Coumarin-3-carboxylic acid
99%
Synonym(s):
2-Oxo-2H-1-benzopyran-3-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H6O4
CAS Number:
Molecular Weight:
190.15
Beilstein:
154276
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
189-192 °C (lit.)
SMILES string
OC(=O)C1=Cc2ccccc2OC1=O
InChI
1S/C10H6O4/c11-9(12)7-5-6-3-1-2-4-8(6)14-10(7)13/h1-5H,(H,11,12)
InChI key
ACMLKANOGIVEPB-UHFFFAOYSA-N
Gene Information
human ... PTPN1(5770)
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shinichi Yamashita et al.
Free radical research, 46(7), 861-871 (2012-04-17)
The radiation-induced reactions of a water-soluble coumarin derivative, coumarin-3-carboxyl acid (C3CA), have been investigated in aqueous solutions by pulse radiolysis with a 35 MeV electron beam, final product analysis following (60)Co γ-irradiations and deterministic model simulations. Pulse radiolysis revealed that
[Generation of hydroxyl radicals and other redox active compounds in the sea water exposed to heat].
A V Chernikov et al.
Biofizika, 47(5), 773-781 (2002-10-26)
The process of heat-induced generation of hydroxyl radicals in seawater was studied using coumarin-3-carboxylic acid as a fluorescent detector of .OH. The rate constants of .OH thermoproduction were determined in the temperature range of 40 to 60 degrees C. The
I H M van Stokkum et al.
Photochemistry and photobiology, 82(2), 380-388 (2006-04-15)
The spectral evolution of three photoactive proteins has been investigated by measuring the fluorescence with good temporal and wavelength resolution and a high signal-to-noise ratio. Upon excitation at 400 nm wild-type (wt) PYP both at neutral pH and in the
B S Creaven et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 84(1), 275-285 (2011-10-15)
Novel Ni(II), Co(II), Zn(II) and Mn(II) complexes of coumarin-3-carboxylic acid (HCCA) were studied at experimental and theoretical levels. The complexes were characterised by elemental analyses, FT-IR, (1)H NMR, (13)C NMR and UV-Vis spectroscopy and by magnetic susceptibility measurements. The binding
Franco Chimenti et al.
Bioorganic & medicinal chemistry letters, 14(14), 3697-3703 (2004-06-19)
A series of coumarin-3-acyl derivatives have been synthesized and investigated for the ability to inhibit selectively monoamine oxidases. The coumarin-3-carboxylic acids, 2a-e, proved to be reversible and selective inhibitors of the MAO-B isoform, displaying pIC(50) values of particular interest: 2a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service