361461
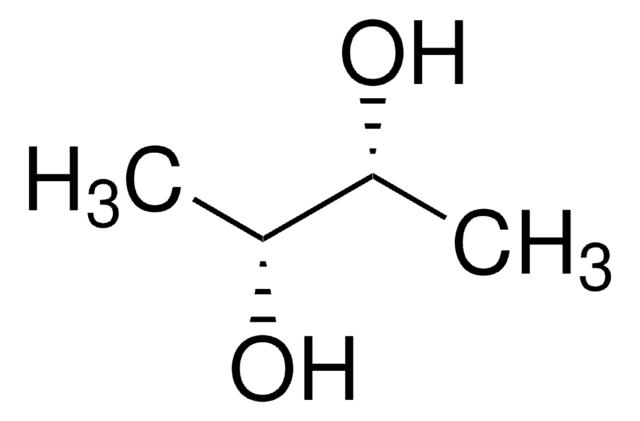
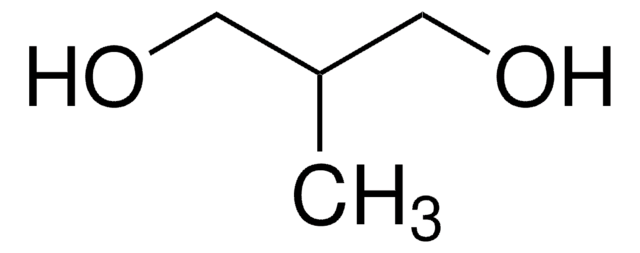
meso-2,3-Butanediol
99%
Synonym(s):
(2R,3S)-2,3-Butanediol, (R,S)-2,3-Butanediol, (erythro-) 2,3-Butanediol, rel-(2R,3S)-2,3-Butanediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
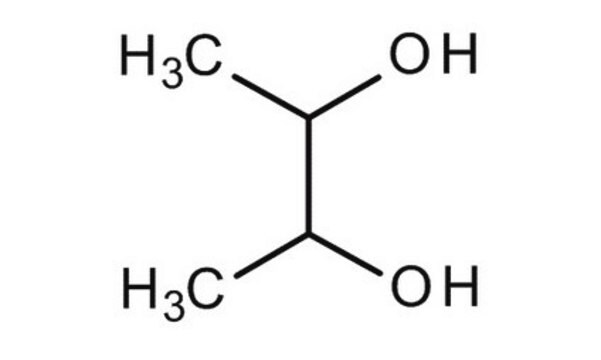
CH3CH(OH)CH(OH)CH3
CAS Number:
Molecular Weight:
90.12
Beilstein:
1718900
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
183-184 °C (lit.)
mp
32-34 °C (lit.)
functional group
hydroxyl
storage temp.
2-8°C
SMILES string
C[C@@H](O)[C@H](C)O
InChI
1S/C4H10O2/c1-3(5)4(2)6/h3-6H,1-2H3/t3-,4+
InChI key
OWBTYPJTUOEWEK-ZXZARUISSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
meso-2,3-Butanediol is a diol. 2,3-Butanediol (2,3-DB) exists in three stereoisomeric forms: dextro, levo and meso. 2,3-DB is a crucial chemical feedstock and has wide applications in industry. Production of meso-2,3-butanediol under low oxygen condition by metabolically engineered Escherichia coli is reported. 2,3-Butanediol can be converted to 1,3-butadiene, which is used in the preparation of synthetic rubber. meso-2,3-Butanediol is the source of production of 2-butanol by isolates of lactic acid bacteria.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
No data available
Flash Point(C)
No data available
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Payam Ghiaci et al.
FEMS microbiology letters, 360(1), 70-75 (2014-09-02)
2-Butanol has been an issue of industries in many areas, for example, biofuel production (as an advanced alternate fuel), fermented beverages, and food (as taste-altering component). Thus, its source of production, the biological pathway, and the enzymes involved are of
Zheng-Jun Li et al.
Applied microbiology and biotechnology, 87(6), 2001-2009 (2010-05-26)
A metabolically engineered Escherichia coli has been constructed for the production of meso-2,3-butanediol (2,3-BD) under low oxygen condition. Genes responsible for 2,3-BD formation from pyruvate were assembled together to generate a high-copy plasmid pEnBD, in which each gene was transcribed
Yuanzhi He et al.
Molecules (Basel, Switzerland), 23(3) (2018-03-23)
(3S)-Acetoin and (2S,3S)-2,3-butanediol are important platform chemicals widely applied in the asymmetric synthesis of valuable chiral chemicals. However, their production by fermentative methods is difficult to perform. This study aimed to develop a whole-cell biocatalysis strategy for the production of
M J Syu
Applied microbiology and biotechnology, 55(1), 10-18 (2001-03-10)
2,3-Butanediol (2,3-BDL), which is very important for a variety of chemical feedstocks and liquid fuels, can be derived from the bioconversion of natural resources. One of its well known applications is the formation of methyl ethyl ketone, by dehydration, which
Hsin-Chih Lai et al.
Journal of leukocyte biology, 92(4), 807-814 (2012-07-18)
The natural compound 2,3-BTD has diverse physiological effects in a range of organisms, including acting as a detoxifying product of liver alcohol metabolism in humans and ameliorating endotoxin-induced acute lung injury in rats. In this study, we reveal that 2,3-BTD
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service