300349
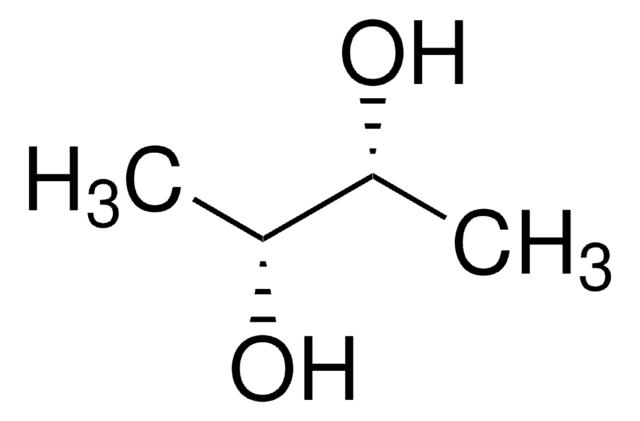
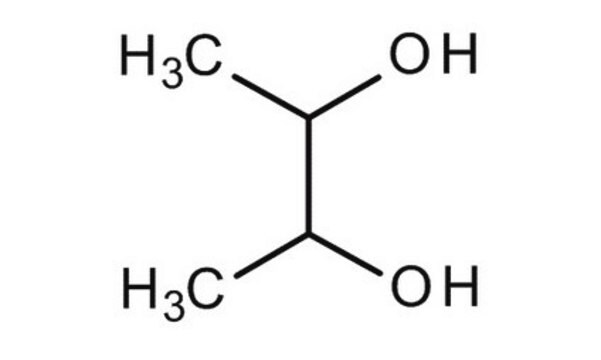
(2S,3S)-(+)-2,3-Butanediol
97%
Synonym(s):
(+)-2,3-Butanediol, (2S,3S)-2,3-Butanediol, (S,S)-2,3-Butanediol, Dextro-2,3-Butanediol, L-(+)-Butane-2,3-diol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3CH(OH)CH(OH)CH3
CAS Number:
Molecular Weight:
90.12
Beilstein:
1718899
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
form:
liquid
Assay:
97%
Recommended Products
Quality Level
Assay
97%
form
liquid
optical activity
[α]20/D +13°, neat
optical purity
ee: 99% (GLC)
refractive index
n20/D 1.433 (lit.)
bp
179-182 °C (lit.)
density
0.987 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
C[C@H](O)[C@H](C)O
InChI
1S/C4H10O2/c1-3(5)4(2)6/h3-6H,1-2H3/t3-,4-/m0/s1
InChI key
OWBTYPJTUOEWEK-IMJSIDKUSA-N
Related Categories
General description
(2S,3S)-2,3-Butanediol ((2S,3S)-2,3-BD) is a chiral compound that can be used as a building block in organic synthesis for the preparation of bioactive molecules.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
185.0 °F - closed cup
Flash Point(C)
85 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Biocatalytic production of (2S, 3S)-2, 3-butanediol from diacetyl using whole cells of engineered Escherichia coli
Li L, et al.
Bioresource Technology, 115, 111-116 (2012)
Yuansheng Xue et al.
Sheng wu gong cheng xue bao = Chinese journal of biotechnology, 27(12), 1742-1748 (2012-04-18)
The production of 2, 3-butanediol and succinic acid by a moderate halophile under anaerobic condition was investigated. This halophile, termed Salinivibrio YS, was isolated from the solid samples collected from Aydingkol Lake. Based on the single factor experiment, the parameters
Yuzhen Zhang et al.
Bioresource technology, 121, 100-104 (2012-08-04)
Pretreatment is necessary for lignocellulose to achieve a highly efficient enzymatic hydrolysis and fermentation. However, coincident with pretreatment, compounds inhibiting microorganism growth are formed. Some tissues or cells, such as thin-walled cells that easily hydrolyze, will be excessively degraded because
Natalia Malfanova et al.
Archives of microbiology, 195(1), 9-17 (2012-09-08)
Twenty endophytic bacteria were isolated from surface-sterilized stems and roots of cucumber plants. After removal of potential siblings and human pathogens, the remaining seven strains were identified based on their 16S rDNA as Pseudomonas fluorescens (2 strains) and P. putida
Chiam Yu Ng et al.
Microbial cell factories, 11, 68-68 (2012-05-30)
2,3-Butanediol is a chemical compound of increasing interest due to its wide applications. It can be synthesized via mixed acid fermentation of pathogenic bacteria such as Enterobacter aerogenes and Klebsiella oxytoca. The non-pathogenic Saccharomyces cerevisiae possesses three different 2,3-butanediol biosynthetic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service