244759
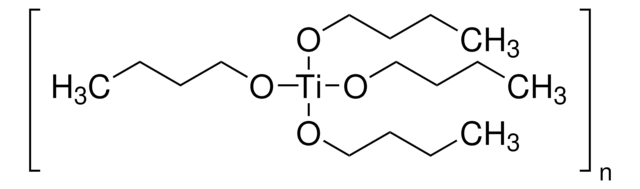
Titanium(IV) ethoxide
technical grade
Synonym(s):
Tetraethyl orthotitanate, Tetraethyl titanate
About This Item
Recommended Products
grade
technical grade
form
liquid
composition
Ti, 19.0-21.0% gravimetric
reaction suitability
core: titanium
reagent type: catalyst
impurities
~20% Tetraisopropyl orthotitanate
refractive index
n20/D 1.505 (lit.)
bp
150-152 °C/10 mmHg (lit.)
density
1.088 g/mL at 25 °C (lit.)
SMILES string
CCO[Ti](OCC)(OCC)OCC
InChI
1S/4C2H5O.Ti/c4*1-2-3;/h4*2H2,1H3;/q4*-1;+4
InChI key
JMXKSZRRTHPKDL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- in the sol-gel synthesis of free-standing ferroelectric lead zirconate titanate nanoparticles
- spherical titania cores, produced either by hydrolysis of Ti(IV) ethoxide droplets in contact with water vapor or
- by preadsorption of the titanium alkoxides into/onto the clay mineral platelets and their subsequent hydrolysis and calcination.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
107.6 °F - closed cup
Flash Point(C)
42 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Mesoporous Oxides and Their Applications to Hydrogen Storage
Titanium dioxide (TiO2) is an important n-type semiconducting material that shows interesting characteristics such as photoswitchable surface wettability, high photocatalytic activity, bistable electrical resistance states and high electron drift mobility.
A Review of Mesoporous TiO2 Thin Films
Thermoelectric Performance of Perovskite-type Oxide Materials
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service