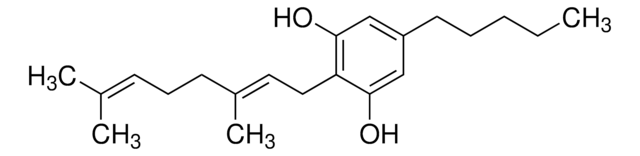
C-144
Cannabidiolic acid solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
(Snap-N-Spike®)
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in acetonitrile
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
cannabis testing
cannabis testing
format
single component solution
shipped in
dry ice
storage temp.
−70°C
InChI
1S/C22H30O4/c1-5-6-7-8-15-12-18(23)20(21(24)19(15)22(25)26)17-11-14(4)9-10-16(17)13(2)3/h11-12,16-17,23-24H,2,5-10H2,1,3-4H3,(H,25,26)/t16-,17+/m0/s1
InChI key
WVOLTBSCXRRQFR-DLBZAZTESA-N
Looking for similar products? Visit Product Comparison Guide
General description
Cannabidiolic acid (CBDA) is one of the main cannabinoids found in plants of the Cannabaceae family, such as hemp and cannabis. It occurs mostly in the leaves of the plant and on decarboxylation in the presence of light or heat forms cannabidiol (CBD). It is known to show anxiolytic, anti-inflammatory, anti-hyperalgesic, antiemetic, neuroprotective, and anticonvulsant activities.
Application
- Determination of four cannabinoids from an oil matrix using reversed-phase high-performance liquid chromatography (RP-HPLC) combined with a diode array detector (DAD)
- Analysis of hemp seed oil samples for identification and estimation of seven major cannabinoids by HPLC coupled to a UV detector
- Separation and determination of CBD and CBDA from hemp oil samples using liquid-liquid extraction and HPLC-DAD
- Multi-analysis of 11 cannabinoids in biomass and extracts of different cannabis varieties by an HPLC-UV method, following the International Conference on Harmonization (ICH) Tripartite Guideline for Validation of Analytical Procedures
- Development and validation of an HPLC-DAD method for the simultaneous analysis of five major neutral and acidic cannabinoids in cannabis and hashish samples
- Gas chromatography-mass spectrometry (GC-MS) analysis of ten main cannabinoids from hemp inflorescences following their silylation and esterification for derivatization
Features and Benefits
- Fully characterized under ISO/IEC 17025 and ISO 17034 accreditation
- Accompanied with a comprehensive Certificate of Analysis (CoA) with data on stability, homogeneity, accuracy of concentration, uncertainty, and traceability
- Rigorously tested through real-time stability studies to ensure accuracy and shelf life
- Gravimetrically prepared using qualified precision balances to ensure minimal uncertainty
- Flame sealed under argon into ampoules for long-term shelf life
- Offered in a convenient, DEA-exempt format to improve laboratory efficiency
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
35.6 °F - closed cup
Flash Point(C)
2.0 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
As the popularity of cannabis-infused products increases, there is a growing need to characterize the type and content of the cannabinoids found in the product. This application demonstrates the ability of the Ascentis Express C18 column to baseline resolve 14 structurally-similar cannabinoids, in under seven minutes, with excellent peak shape.
Tetrahydrocannabinolic acid A solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material.
The cannabinoids found in the Cannabis plant commonly referred to as marijuana, have grown in popularity for treating a variety of ailments from arthritis, glaucoma, and chronic pain to malnutrition, multiple sclerosis, and cancer.
Protocols
Potency testing in marijuana-infused edibles is an important problem that analytical labs are facing due to the complexity of the involved matrices. Concentration of active ingredients in these edibles can range from a few parts per million to 3.5 parts per thousand. This application demonstrates the extraction and HPLC-UV analysis of the active compounds.
As the popularity of cannabis-infused products increases, there is a growing need to characterize the type and content of the cannabinoids found in the product. This application demonstrates the ability of the Ascentis Express C18 column to baseline resolve 14 structurally-similar cannabinoids, in under seven minutes, with excellent peak shape.
Rapid potency testing of marijuana-infused edibles using LC/MS on a biphenyl stationary phase detected eleven cannabinoids.
Tetrahydrocannabinolic acid A solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Cannabichromenic Acid (CBCA) solution, 1.0 mg/mL in acetonitrile, certified reference material, ampule of 1 mL
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service