P22370
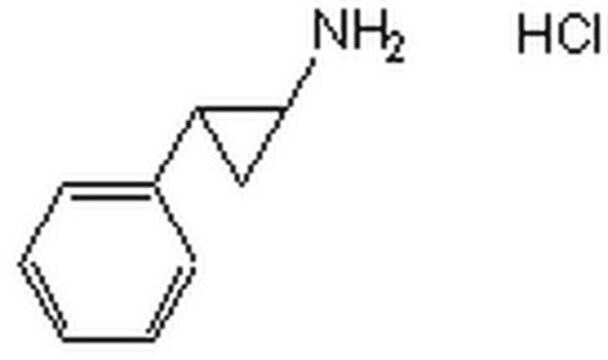
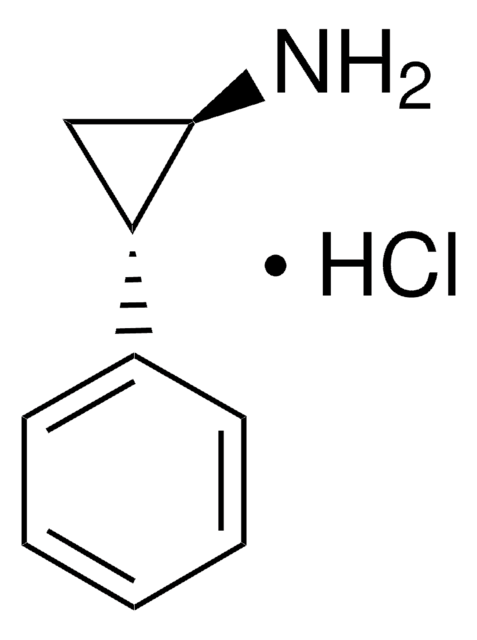
trans-2-Phenylcyclopropylamine hydrochloride
97%
Synonym(s):
Tranylcypromine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H5C3H4NH2·HCl
CAS Number:
Molecular Weight:
169.65
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
optical activity
[α]/D −1 to +1.0°, c = 1 in H2O
mp
162-169 °C (lit.)
storage temp.
2-8°C
SMILES string
Cl.N[C@@H]1C[C@H]1c2ccccc2
InChI
1S/C9H11N.ClH/c10-9-6-8(9)7-4-2-1-3-5-7;/h1-5,8-9H,6,10H2;1H/t8-,9+;/m0./s1
InChI key
ZPEFMSTTZXJOTM-OULXEKPRSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Biochem/physiol Actions
Non-selective MAO-A/B inhibitor.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kaisa A Salminen et al.
Drug metabolism and disposition: the biological fate of chemicals, 43(12), 1891-1904 (2015-09-25)
The cytochrome P450 2C19 (CYP2C19) enzyme plays an important role in the metabolism of many commonly used drugs. Relatively little is known about CYP2C19 inhibitors, including compounds of natural origin, which could inhibit CYP2C19, potentially causing clinically relevant metabolism-based drug
James M Hill et al.
Science translational medicine, 6(265), 265ra169-265ra169 (2014-12-05)
Herpesviruses are highly prevalent and maintain lifelong latent reservoirs, thus posing challenges to the control of herpetic disease despite the availability of antiviral pharmaceuticals that target viral DNA replication. The initiation of herpes simplex virus infection and reactivation from latency
Ramakrishna Nirogi et al.
Chemico-biological interactions, 230, 9-20 (2015-02-07)
The objective of the study was to evaluate the metabolism dependent inhibition of CYP2B6 catalyzed bupropion hydroxylation in human liver microsomes by monoamine oxidase (MAO) inhibitors and to predict the drug-drug interaction potential of monoamine oxidase inhibitors as perpetrators of
Yuki Ogawa et al.
European journal of pediatrics, 174(4), 509-518 (2014-09-25)
This study aimed to determine the population pharmacokinetics of doxapram in low-birth-weight (LBW) infants. A total of 92 serum concentration measurements that were obtained from 34 Japanese neonates were analyzed using nonlinear mixed-effect modeling (NONMEM). Estimates generated by NONMEM indicated
Christine R Klaus et al.
The Journal of pharmacology and experimental therapeutics, 350(3), 646-656 (2014-07-06)
EPZ-5676 [(2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1r,3S)-3-(2-(5-(tert-butyl)-1H-benzo[d]imidazol-2-yl)ethyl)cyclobutyl)(isopropyl)amino)methyl)tetrahydrofuran-3,4-diol], a small-molecule inhibitor of the protein methyltransferase DOT1L, is currently under clinical investigation for acute leukemias bearing MLL-rearrangements (MLL-r). In this study, we evaluated EPZ-5676 in combination with standard of care (SOC) agents for acute leukemias as well
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service