690414
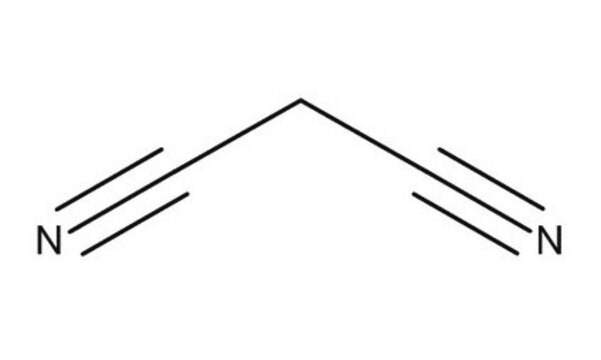
Malononitrile
Arxada quality, ≥99.0% (calculated, GC, KF)
Synonym(s):
Dicyanomethane
About This Item
Recommended Products
Quality Level
Assay
≥99.0% (calculated, GC, KF)
form
liquid
quality
Arxada quality
manufacturer/tradename
Arxada AG
impurities
≤0.10% water
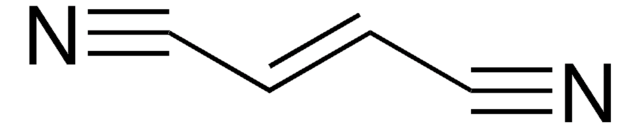
≤0.50% (E)-2-butenedinitrile
≤0.50% (Z)-2-butenedinitrile
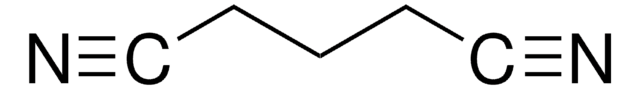
≤0.50% butanedinitrile
bp
220 °C (lit.)
mp
30-32 °C (lit.)
density
1.049 g/mL at 25 °C (lit.)
functional group
nitrile
storage temp.
2-8°C
SMILES string
N#CCC#N
InChI
1S/C3H2N2/c4-2-1-3-5/h1H2
InChI key
CUONGYYJJVDODC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Some of the reactions where malononitrile is used as a reactant are:
- Synthesis of 2-pyran-4-ylidene-malononitrile (PM) based red light emitting polymers.
- Synthesis of polysubstituted dihydropyridines.
- Synthesis of various chromene derivatives upon treating with salicylic aldehydes.
- Synthesis of triselenium dicyanide by treating it with selenium dioxide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
186.8 °F - closed cup
Flash Point(C)
86 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
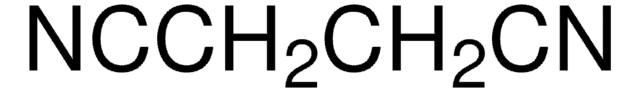
![2-[Bis(methylthio)methylene]malononitrile 97%](/deepweb/assets/sigmaaldrich/product/structures/144/342/6a420594-3bce-4984-a8b7-5bf2a92d6a97/640/6a420594-3bce-4984-a8b7-5bf2a92d6a97.png)