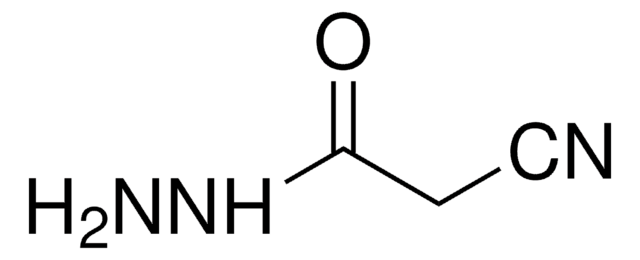
108421
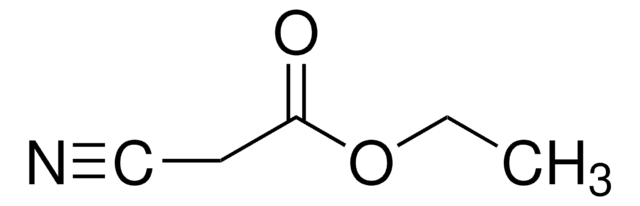
Methyl cyanoacetate
99%
Synonym(s):
Methyl 2-cyanoacetate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
NCCH2COOCH3
CAS Number:
Molecular Weight:
99.09
Beilstein:
773945
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39031513
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3.41 (vs air)
Quality Level
vapor pressure
0.2 mmHg ( 20 °C)
Assay
99%
refractive index
n20/D 1.417 (lit.)
bp
204-207 °C (lit.)
mp
−13 °C (lit.)
density
1.123 g/mL at 25 °C (lit.)
functional group
ester
nitrile
SMILES string
COC(=O)CC#N
InChI
1S/C4H5NO2/c1-7-4(6)2-3-5/h2H2,1H3
InChI key
ANGDWNBGPBMQHW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Methyl cyanoacetate is the intermediate product in pharmaceutical organic synthesis as well as in the synthesis of some biologically active compounds used in agriculture. It undergoes calcite or fluorite catalyzed Knövenagel condensation with aromatic aldehydes, giving the corresponding arylidenemalononitriles and (E)-α-cyanocinnamic esters.
Application
Methyl cyanoacetate may be used in the synthesis of various 1,2,5-tricarbonyl compounds.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Calcite and fluorite as catalyst for the Knovenagel condensation of malononitrile and methyl cyanoacetate under solvent-free conditions.
Wada S and Suzuki H.
Tetrahedron Letters, 44(2), 399-401 (2003)
Conformational isomerism in methyl cyanoacetate: A combined matrix-isolation infrared spectroscopy and molecular orbital study.
Reva ID, et al
Physical Chemistry Chemical Physics, 3(19), 4235-4241 (2011)
Construction of 1, 2, 5-Tricarbonyl Compounds using Methyl Cyanoacetate as a Glyoxylate Anion Synthon Combined with Copper (I) Iodide-Catalyzed Aerobic Oxidation.
Kim SH, et al.
Advanced Synthesis & Catalysis, 353(18), 3335-3339 (2011)
Aerobic dissipation of the novel cyanoacrylate fungicide phenamacril in soil and sludge incubations.
Søren S Donau et al.
Chemosphere, 233, 873-878 (2019-07-26)
The cyanoacrylate ethyl (2Z)-3-amino-2-cyano-3-phenylacrylate (phenamacril), has been introduced as an effective agent against several fungi species belonging to the Fusarium genus. However, in current literature, knowledge about the environmental behavior of this fungicide is limited and there are no data
Yulia Kaluzhny et al.
Alternatives to laboratory animals : ATLA, 43(2), 101-127 (2015-05-23)
The 7th Amendment to the EU Cosmetics Directive and the EU REACH Regulation have reinforced the need for in vitro ocular test methods. Validated in vitro ocular toxicity tests that can predict the human response to chemicals, cosmetics and other
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service