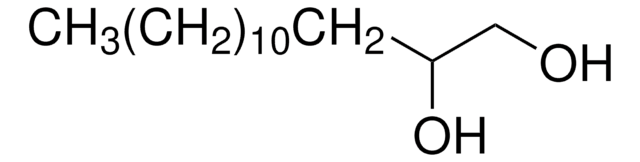213705
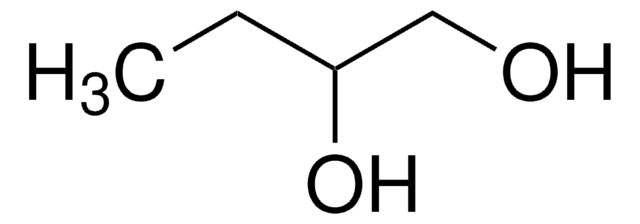
1,2-Octanediol
98%
Synonym(s):
(±)-Octane-1,2-diol, 1,2-Dihydroxyoctane, 1,2-Octylene glycol, 7,8-Dihydroxyoctane, Caprylyl glycol, n-Octane-1,2-diol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
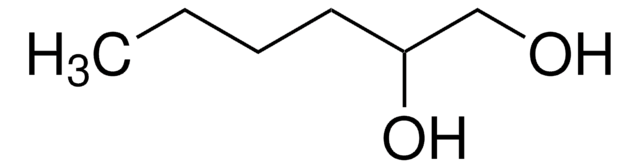
CH3(CH2)5CH(OH)CH2OH
CAS Number:
Molecular Weight:
146.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
>1 (vs air)
Quality Level
Assay
98%
form
solid
bp
131-132 °C/10 mmHg (lit.)
mp
36-38 °C (lit.)
functional group
hydroxyl
SMILES string
CCCCCCC(O)CO
InChI
1S/C8H18O2/c1-2-3-4-5-6-8(10)7-9/h8-10H,2-7H2,1H3
InChI key
AEIJTFQOBWATKX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1,2-Octanediol is a potential pediculicide and is useful for treating head louse infestation clinically.
Application
1,2-Octanediol was used as organic modifier to improve the HPLC separation of organic acids and bases. It was also used in preparation of halohydrin palmitates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ian F Burgess et al.
PloS one, 7(4), e35419-e35419 (2012-04-24)
Interest in developing physically active pediculicides has identified new active substances. The objective was to evaluate a new treatment for clinical efficacy. We describe the selection of 1,2-octanediol as a potential pediculicide. Clinical studies were community based. The main outcome
Shelly Li et al.
Journal of chromatography. A, 964(1-2), 91-98 (2002-08-30)
A straight-chain alcohol or diol additive in the mobile phase was used to modify and improve the HPLC separation of organic acids and bases. Incorporation of 2% 1-butanol, 1% 1,2-hexanediol, or 0.25% 1,2-octanediol into an aqueous mobile phase greatly improved
Mireia Oromí-Farrús et al.
Molecules (Basel, Switzerland), 14(10), 4275-4283 (2009-11-20)
Preparation of (S)-1-chloro-2-octanol and (S)-1-bromo-2-octanol was carried out by the enzymatic hydrolysis of halohydrin palmitates using biocatalysts. Halohydrin palmitates were prepared by various methods from palmitic acid and 1,2-octanediol. A tandem hydrolysis was carried out using lipases from Candida antarctica
Kelli L Hvorecny et al.
Structure (London, England : 1993), 25(5), 697-707 (2017-04-11)
Pseudomonas aeruginosa secretes an epoxide hydrolase with catalytic activity that triggers degradation of the cystic fibrosis transmembrane conductance regulator (CFTR) and perturbs other host defense networks. Targets of this CFTR inhibitory factor (Cif) are largely unknown, but include an epoxy-fatty
Chromatograms
suitable for GCsuitable for GCGlobal Trade Item Number
| SKU | GTIN |
|---|---|
| 213705-50G | 4061838772947 |
| 213705-10G | 4061838772930 |
| 213705-1KG |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service