213691
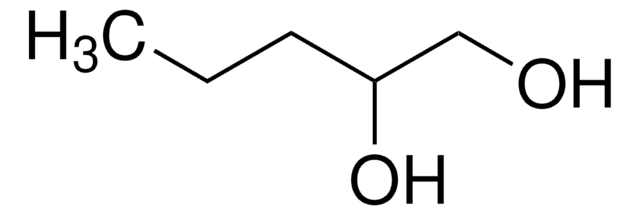
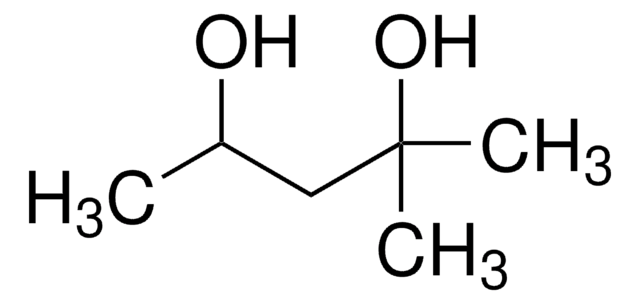
1,2-Hexanediol
98%
Synonym(s):
1,2-Dihydroxyhexane, 1,2-Hexyleneglycol, 5,6-Dihydroxyhexane, DL-1,2-Hexanediol, Dermasoft Hexiol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)3CH(OH)CH2OH
CAS Number:
Molecular Weight:
118.17
Beilstein:
1719244
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.442 (lit.)
bp
223-224 °C (lit.)
density
0.951 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
CCCCC(O)CO
InChI
1S/C6H14O2/c1-2-3-4-6(8)5-7/h6-8H,2-5H2,1H3
InChI key
FHKSXSQHXQEMOK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1,2-Hexanediol acts as cosurfactant, used for modifying the sodium dodecyl sulfate micelles. Solubility of 1,2-hexanediol in supercritical CO2 has been reported. It has a tendency of self-association to form micelle-like aggregates.
Application
1,2-Hexanediol can be used in the ruthenium-catalyzed synthesis of oxazolidin-2-ones from urea. It can undergo ruthenium-hydride catalyzed dehydrative coupling with anilines to form substituted indole and quinoline products.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
251.6 °F - closed cup
Flash Point(C)
122 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
(Enantio) selective Hydrogen Autotransfer: Ruthenium?Catalyzed Synthesis of Oxazolidin?2?ones from Urea and Diols.
Pe?a?Lopez M, et al.
Angewandte Chemie (International Edition in English), 55(27), 7826-7830 (2016)
Catalytic Synthesis of Substituted Indoles and Quinolines from the Dehydrative C?H Coupling of Arylamines with 1, 2-and 1, 3-Diols.
Lee H and Yi C S
Organometallics, 35(11), 1973-1977 (2016)
Solubilities of (1-hexanol, or 1, 2-hexanediol, or 2-hydroxypropanoic acid ethyl ester, or 2-hydroxyhexanoic acid ethyl ester) in supercritical CO2.
Chylinski K and Gregorowicz J.
The Journal of Chemical Thermodynamics, 30(9), 1131-1140 (1998)
Sooho Yeo et al.
Pharmaceutics, 13(1) (2021-01-01)
Adenosine (AD), which is used for treating wrinkles, exhibits poor skin permeation. The aim of the present study was to develop a cross-linked silicone-based cellulose elastomer as an elastic artificial skin for the treatment of skin wrinkles, a biocompatible lipid-based
R G Alany et al.
International journal of pharmaceutics, 196(2), 141-145 (2000-03-04)
The aim of the current study was to investigate the effect of different co-surfactants on the phase behaviour of the pseudoternary system water:ethyl oleate:nonionic surfactant blend (sorbitan monolaurate/polyoxyethylene 20 sorbitan mono-oleate). Four aliphatic alcohols (1-propanol, 1-butanol, 1-hexanol and 1-octanol) and
Chromatograms
suitable for GCsuitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service