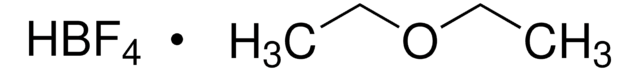90520
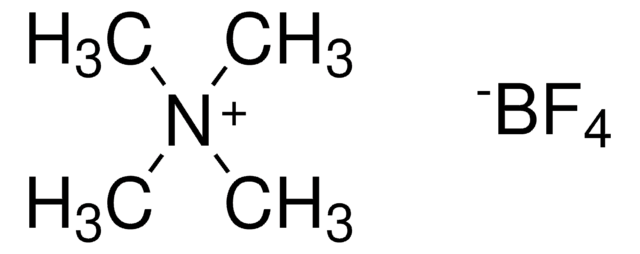
Triethyloxonium tetrafluoroborate
≥97.0% (T)
Synonym(s):
Et3OBF4, Meerwein′s reagent, Triethyloxonium fluoroborate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C2H5)3O(BF4)
CAS Number:
Molecular Weight:
189.99
Beilstein:
3598090
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (T)
form
crystals
contains
1-3% ether as stabilizer
solubility
methylene chloride: 20 mg/mL, clear, colorless
functional group
ether
storage temp.
2-8°C
SMILES string
F[B-](F)(F)F.CC[O+](CC)CC
InChI
1S/C6H15O.BF4/c1-4-7(5-2)6-3;2-1(3,4)5/h4-6H2,1-3H3;/q+1;-1
InChI key
IYDQMLLDOVRSJJ-UHFFFAOYSA-N
Application
Triethyloxonium tetrafluoroborate can be used:
- To prepare amino esters by reacting with lactams followed by hydrolysis.
- In the preparation of substituted imidazolines from aziridines and nitriles via [3+2]-cycloaddition reaction.
- For the N-alkylation of a series of N-arylsulfonyl-α-amino acid methyl esters having variable substituents at 4th position of the sulfonamide aromatic ring.
Other Notes
Powerful ethylating agent; Esterification of acids; Modifies carboxyl residues in proteins
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of substituted imidazolines via [3+ 2]-cycloaddition of aziridines with nitriles
Prasad BAB, et al.
Tetrahedron Letters, 45(6), 1137-1141 (2004)
N-Alkylation of N-arylsulfonyl-α-amino acid methyl esters by trialkyloxonium tetrafluoroborates
De Marco R, et al.
Tetrahedron, 67(50), 9708-9714 (2011)
H. Meerwein et al.
Angewandte Chemie (International Edition in English), 72, 927-927 (1960)
A mild and facile route to ω-amino esters
Menezes, R and Smith, MB
Synthetic Communications, 18(14), 1625-1636 (1988)
The nature of amino acid side chains which are critical for the activity of lysozyme.
S M Parsons et al.
Biochemistry, 8(2), 700-712 (1969-02-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service