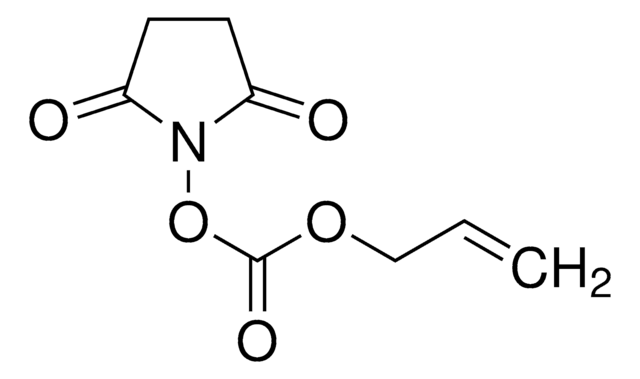
730300
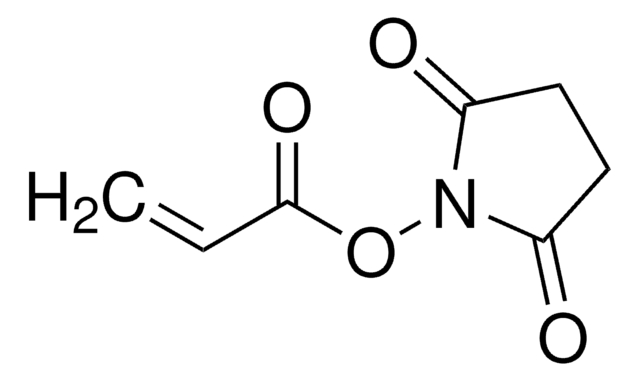
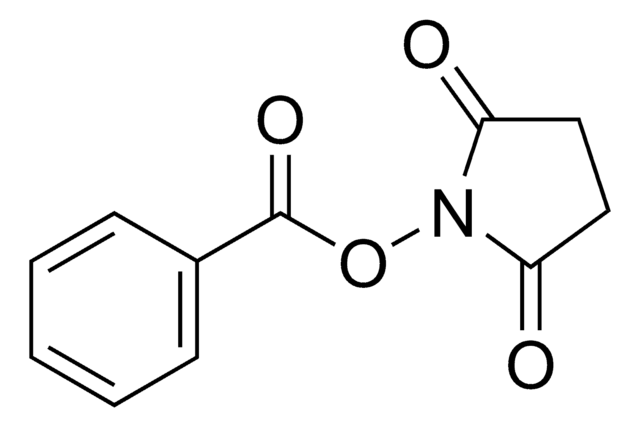
Methacrylic acid N-hydroxysuccinimide ester
98%
Synonym(s):
N-(Methacryloxy)succinimide, N-(Methacryloyloxy)succinimide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H9NO4
CAS Number:
Molecular Weight:
183.16
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
solid
mp
101-105 °C
storage temp.
2-8°C
SMILES string
CC(=C)C(=O)ON1C(=O)CCC1=O
InChI
1S/C8H9NO4/c1-5(2)8(12)13-9-6(10)3-4-7(9)11/h1,3-4H2,2H3
InChI key
ACGJEMXWUYWELU-UHFFFAOYSA-N
General description
Methacrylic acid N-hydroxysuccinimide ester(NHS-MA) belongs to the class of reactive monomers known as N-hydroxysuccinimide (NHS) esters. It is commonly used for functionalizing biomolecules through amine-reactive coupling reactions infields such as bioconjugation, protein labeling, and peptide synthesis. It also serves as a crosslinking agent or linker molecule for the conjugation of drugs or targeting ligands to carrier materials.
Application
Methacrylic acid N-hydroxysuccinimide ester can be used:
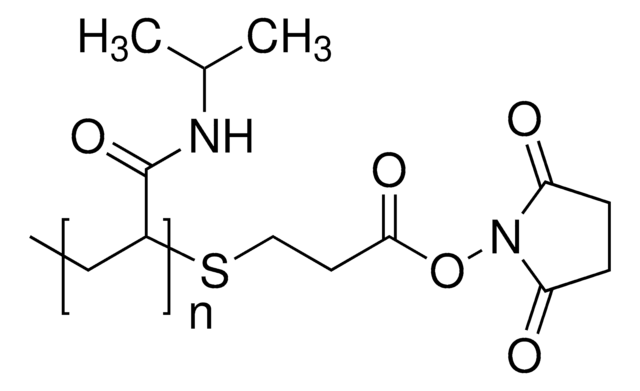
- As a monomer to prepare degradable amphiphilic diblock copolymer microparticles via RAFT polymerization, for low pH-triggered drug delivery. NHS-MA can shield the drug molecule from degradation, enhance its solubility, and improve its pharmacokinetic properties.
- For the surface functionalization of poly-ε-caprolactone (PCL) scaffolds used for tissue engineering. NHS groups are used to couple with chitosan of various molecular weights.
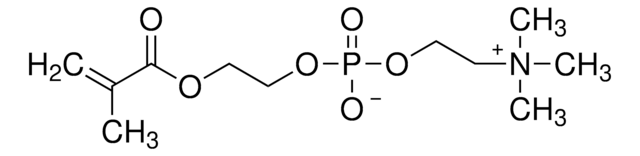
- To prepare biocompatible polymer hydrogel for enzymatic biofuel cells. The hydrogel can serve as an enzyme-immobilizing matrix for enzymatic bioelectrodes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Theato, P.
Journal of Polymer Science Part A: Polymer Chemistry, 46, 6677-6677 (2008)
Xiaolong Zhang et al.
Biomacromolecules, 17(3), 778-787 (2016-01-23)
It is important to synthesize materials to recapitulate critical functions of biological systems for a variety of applications such as tissue engineering and regenerative medicine. The purpose of this study was to synthesize a chimeric hydrogel as a promising extracellular
Myriam G Tardajos et al.
Carbohydrate polymers, 191, 127-135 (2018-04-18)
Tissue engineering (TE) approaches often employ polymer-based scaffolds to provide support with a view to the improved regeneration of damaged tissues. The aim of this research was to develop a surface modification method for introducing chitosan as an antibacterial agent
Cori K Cahoon et al.
Proceedings of the National Academy of Sciences of the United States of America, 114(33), E6857-E6866 (2017-08-02)
The synaptonemal complex (SC), a structure highly conserved from yeast to mammals, assembles between homologous chromosomes and is essential for accurate chromosome segregation at the first meiotic division. In
Huan Peng et al.
Biomacromolecules, 17(11), 3619-3631 (2016-09-20)
This paper reports a facile approach for encapsulation of enzymes in nanogels. Our approach is based on the use of reactive copolymers able to get conjugated with enzyme and build 3D colloidal networks or biohybrid nanogels. In a systematic study
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service