All Photos(1)
About This Item
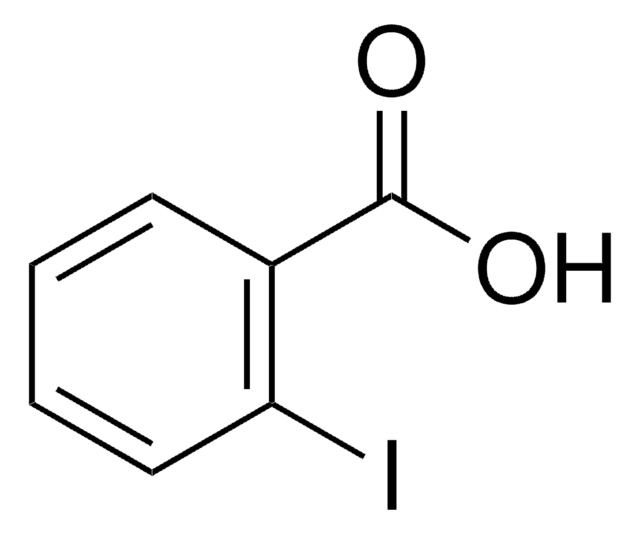
Linear Formula:
IC6H4CH2CO2H
CAS Number:
Molecular Weight:
262.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
116-119 °C (lit.)
functional group
carboxylic acid
iodo
SMILES string
OC(=O)Cc1ccccc1I
InChI
1S/C8H7IO2/c9-7-4-2-1-3-6(7)5-8(10)11/h1-4H,5H2,(H,10,11)
InChI key
IUHXGZHKSYYDIL-UHFFFAOYSA-N
General description
2-Iodophenylacetic acid is a substituted acetic acid that can be prepared from 2-iodobenzyl cyanide. It undergoes palladium-catalyzed reaction with allenes to form 1,3-butadienes. 2-Iodophenylacetic acid also undergoes photolysis in carbon tetrachloride to form the corresponding chloro compound.
Application
2-Iodophenylacetic acid may be used in the preparation of:
- 2-iodophenylacetyl chloride
- 3,4-dimethoxyphenyl 2-iodophenylacetate
- N-{2-[2-(2-iodophenyl)-acetylamino]-ethyl}-2-(3,4- dichlorophenyl)-acetamide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of benzo[c]chromen-6-ones via novel cyclic aryl?Pd (II)?ester enolate intermediates.
Taylor SR, et al.
Tetrahedron, 63(45), 10889-10895 (2007)
Pd?Catalyzed Reactions of Allenylphosphonates and Related Allenes with Functionalized 2?Iodophenols, 2?Iodobenzoic Acid, and 2?Iodobenzyl Alcohol Leading to Functionalized Benzofurans, Isocoumarins, and Benzopyrans.
Pavan MP, et al.
European Journal of Organic Chemistry, 5927-5940 (2009)
Photochemical synthesis of aromatic chloro compounds from aromatic iodo compounds.
The Journal of Organic Chemistry, 35(2), 528-529 (1970)
Regioselective Synthesis of Isoquino [1,2-b][3] benzazepines (Homoprotoberberines) through 11-Membered-Ring Stilbene Lactams Obtained by Radical Macrocyclization.
Rodriguez G, et al.
The Journal of Organic Chemistry, 64(13), 4830-4833 (1999)
Jonathan M Fitzsimmons et al.
Medicinal chemistry, 1(102) (2011-12-25)
Eight halogenated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service