365815
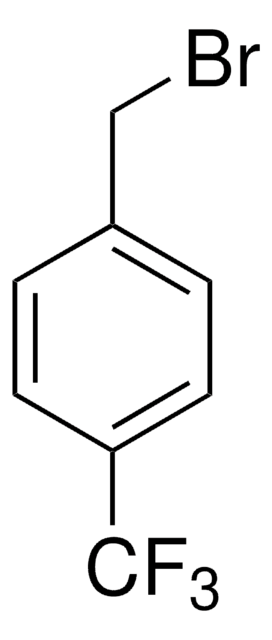
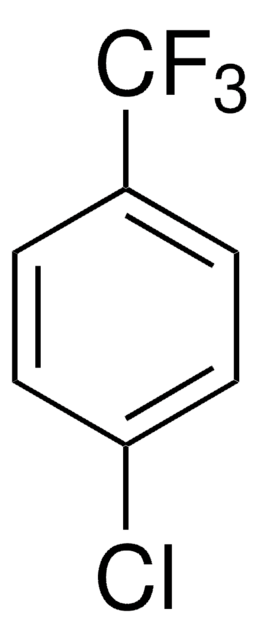
4-(Trifluoromethyl)benzyl chloride
98%
Synonym(s):
α′-Chloro-α,α,α-trifluoro-p-xylene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3C6H4CH2Cl
CAS Number:
Molecular Weight:
194.58
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.464 (lit.)
bp
68 °C/12 mmHg (lit.)
density
1.315 g/mL at 25 °C (lit.)
functional group
chloro
fluoro
SMILES string
FC(F)(F)c1ccc(CCl)cc1
InChI
1S/C8H6ClF3/c9-5-6-1-3-7(4-2-6)8(10,11)12/h1-4H,5H2
InChI key
MCHDHQVROPEJJT-UHFFFAOYSA-N
Related Categories
General description
Electrochemical reduction of 4-(trifluoromethyl)benzyl chloride catalyzed by Co(salen) (H2salen, N,N′-bis(salicylidene)-ethane-1,2-diamine) in acetonitrile is reported.
Application
4-(Trifluoromethyl)benzyl chloride may be used in the synthesis of novel series of dithiocarbamates, via reaction with sodium salts of N,N-disubstituted dithiocarbamic acids.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mechanism of the electrochemical reduction of benzyl chlorides catalysed by Co (salen).
Isse AA, et al.
Journal of Electroanalytical Chemistry, 444(2), 241-245 (1992)
Mehlika D Altıntop et al.
Archiv der Pharmazie, 346(8), 571-576 (2013-07-25)
In the present paper, a novel series of dithiocarbamates was synthesized via the treatment of 4-(trifluoromethyl)benzyl chloride with appropriate sodium salts of N,N-disubstituted dithiocarbamic acids. The chemical structures of the compounds were elucidated by (1) H NMR, mass spectral data
Nicholas R Oranzi et al.
Analytical chemistry, 91(6), 4092-4099 (2019-02-27)
Quantitation of the serum concentration of 25-hydroxyvitamin D is a high-demand assay that suffers from long chromatography time to separate 25-hydroxyvitamin D from its inactive epimer; however, ion mobility spectrometry can distinguish the epimer pair in under 30 ms due
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service